Hold down the T key for 3 seconds to activate the audio accessibility mode, at which point you can click the K key to pause and resume audio. Useful for the Check Your Understanding and See Answers.
Lesson 1: Kinetics of Reactions
Part b: Factors Affecting Reaction Rates
Part a:
Reaction Rates
Part b: Factors Affecting Reaction Rates
Part c:
The Collision Model of Reactions
Part d:
Rate Equations
Part e:
Reaction Mechanisms
Observing Reaction Rates
A reaction rate refers to the rate at which the concentration of a reactant or a product changes over a certain time interval. The equation for the rate of reaction for a reactant is
Rate = ∆[Reactant] / ∆Time
(Rate equals the change in the reactant concentration divided by the time change)
 A Chemistry student will often perform labs to compare the reaction rate under a set of controlled conditions. Since there are a collection of visible indicators of a reaction occurring, observations can be made to compare the rates under varying conditions. Such visible indicators may include the rate at which bubbles are produced by the reaction, the rate at which a colored product is produced, and the rate at which energy is released, temperature rises, or flames are produced. By carefully controlling variables and observing the rate indicators, a careful lab student can identify how a variable may or may not affect a reaction rate. On this page, we will look at four different variables that affect reaction rates.
A Chemistry student will often perform labs to compare the reaction rate under a set of controlled conditions. Since there are a collection of visible indicators of a reaction occurring, observations can be made to compare the rates under varying conditions. Such visible indicators may include the rate at which bubbles are produced by the reaction, the rate at which a colored product is produced, and the rate at which energy is released, temperature rises, or flames are produced. By carefully controlling variables and observing the rate indicators, a careful lab student can identify how a variable may or may not affect a reaction rate. On this page, we will look at four different variables that affect reaction rates.
Concentration of Reactants
 The first variable that impacts the reaction rate is the concentration of the reactants. Reaction rates are higher with higher reactant concentrations. We learned in Lesson 1a that the rate of a reaction decreases with time (with rare exceptions). A reaction begins with a relatively high rate, but the rate gradually decreases with time. As a reaction proceeds, the concentration of a reactant decreases as it is converted to products. This decrease in reactant concentration results in a decrease in the reaction rate.
The first variable that impacts the reaction rate is the concentration of the reactants. Reaction rates are higher with higher reactant concentrations. We learned in Lesson 1a that the rate of a reaction decreases with time (with rare exceptions). A reaction begins with a relatively high rate, but the rate gradually decreases with time. As a reaction proceeds, the concentration of a reactant decreases as it is converted to products. This decrease in reactant concentration results in a decrease in the reaction rate.
Like most metals, zinc reacts with acid. When Zn is added to an aqueous solution of hydrochloric acid, the following single replacement reaction occurs:
Zn(s) + 2 HCl(aq) → ZnCl2(aq) + H2(g)
There are at least two indicators of the reaction. The reaction is exothermic, releasing heat to the surroundings and increasing its temperature. Hydrogen gas bubbles are generated and can be seen bubbling out of the solution.
Let’s suppose the following experiment is performed. The same amount of zinc is added to three beakers of aqueous HCl having three different molar concentrations – 0.1 M, 0.5 M, and 1.0 M. What would we observe?
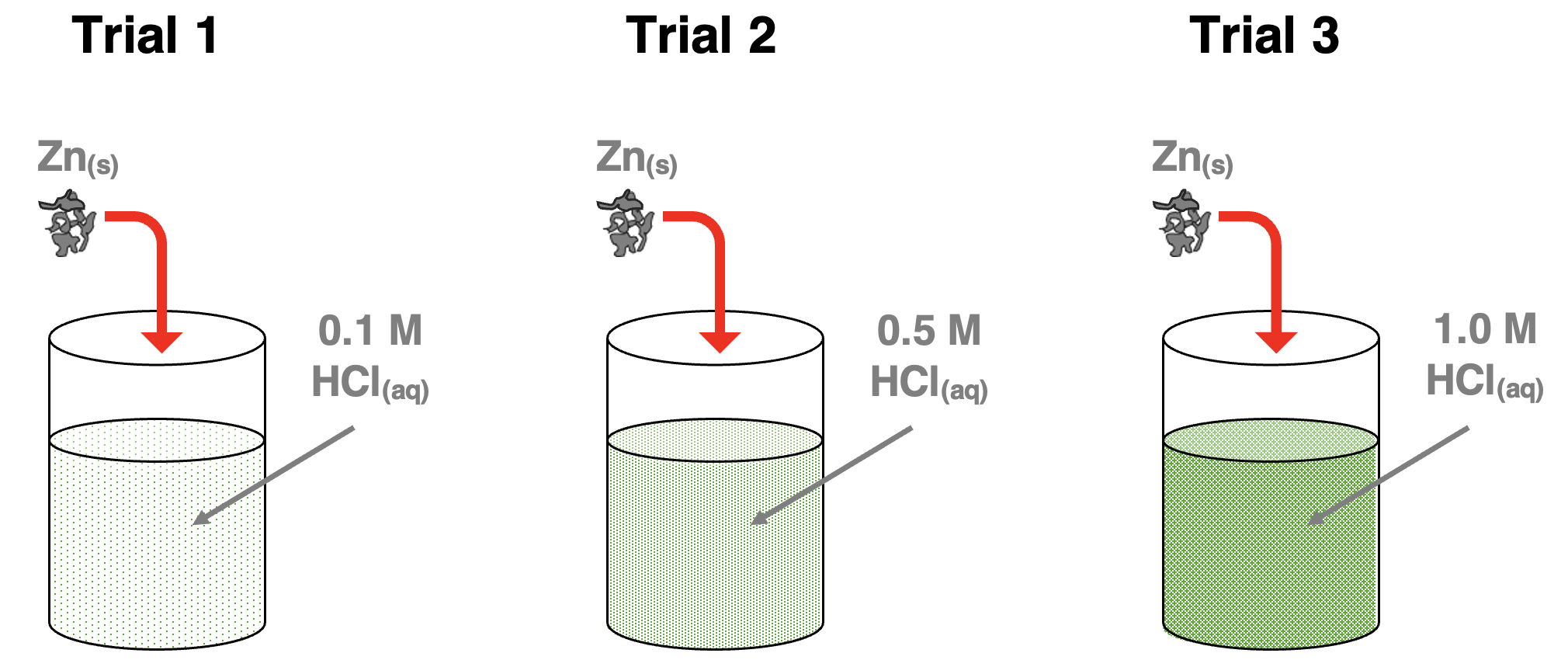
 Since reaction rates increase with increasing concentrations, we would observe the indicators of the greatest rate for Trial 3 and the lowest rate for Trial 1. We would observe that during the early stages of the reaction, the bubbles of H2 gas are most vigorously generated in Trial 3 and least vigorously generated in Trial 1. And if we lightly touched the sides of the beakers after 5 seconds we would notice that the beaker in Trial 3 is significantly warmer than the beaker in Trial 2 and Trial 1. These indicators of the rate of the reaction would confirm the claim that reaction rate increases with increasing reactant concentration.
Since reaction rates increase with increasing concentrations, we would observe the indicators of the greatest rate for Trial 3 and the lowest rate for Trial 1. We would observe that during the early stages of the reaction, the bubbles of H2 gas are most vigorously generated in Trial 3 and least vigorously generated in Trial 1. And if we lightly touched the sides of the beakers after 5 seconds we would notice that the beaker in Trial 3 is significantly warmer than the beaker in Trial 2 and Trial 1. These indicators of the rate of the reaction would confirm the claim that reaction rate increases with increasing reactant concentration.
Temperature
Temperature is a second variable that has a noticeable effect upon reaction rates. A common lab experiment involves the dissolving of an Alka seltzer tablet in water at varying temperatures. A reaction occurs that produces carbon dioxide gas - a visible indicator of the reaction. The Alka seltzer tablet contains both reactants in solid form – citric acid and sodium bicarbonate. As the tablet dissolves, the two reactants are freed up to react with each other in the aqueous state:
H3C6H5O7(aq) + 3 NaHCO3(aq) → 3 H2O(l) + 3 CO2(g) + Na3C6H5O7(aq)
Let’s suppose the following experiment is performed. An Alka seltzer tablet is added to a beaker containing the same amount of water at varying temperatures – 10°C, 30°C, and 70°C. What would we observe?
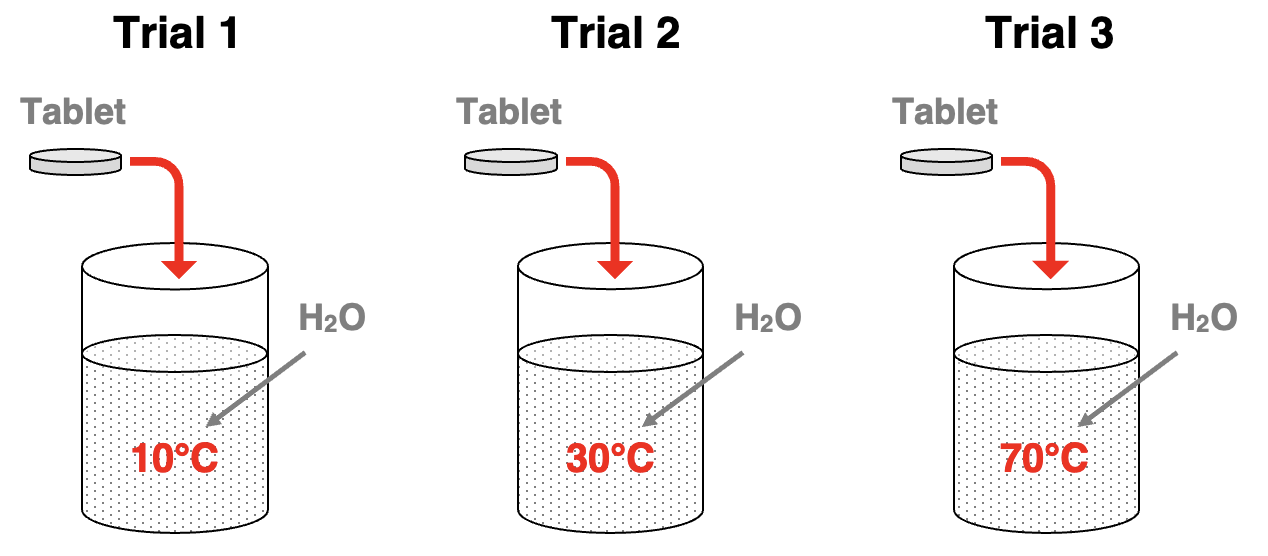
 Since reaction rates increase with increasing temperatures, we would observe the indicators of the greatest rate for Trial 3 and the lowest rate for Trial 1. We would observe that during the early stages of the reaction, the bubbles of CO2 gas are most vigorously generated in Trial 3 and least vigorously generated in Trial 1. Both the dissolving process and the reaction between the two reactants would occur faster at the higher temperatures. The observations of the CO2 gas bubbles confirms the claim that reaction rate increases with increasing temperature.
Since reaction rates increase with increasing temperatures, we would observe the indicators of the greatest rate for Trial 3 and the lowest rate for Trial 1. We would observe that during the early stages of the reaction, the bubbles of CO2 gas are most vigorously generated in Trial 3 and least vigorously generated in Trial 1. Both the dissolving process and the reaction between the two reactants would occur faster at the higher temperatures. The observations of the CO2 gas bubbles confirms the claim that reaction rate increases with increasing temperature.
 The general rule of thumb is that reaction rates will double for every 10°C increase in temperature. It is common practice to refrigerate food to prevent it from spoiling. Food spoils due to a variety of biochemical processes. These processes include oxidation of fats and oils, hydrolysis of fats and proteins, enzymatic browning, and microbial growth. Each of these undesired processes will occur at lower rates when the food is stored in a 5°C refrigerator instead of a 25°C cabinet. The 20°C decrease in temperature would cause the rates of these reactions to be about four times lower. Food preservation through the use of refrigerators is another example of Chemistry for Better Living.
The general rule of thumb is that reaction rates will double for every 10°C increase in temperature. It is common practice to refrigerate food to prevent it from spoiling. Food spoils due to a variety of biochemical processes. These processes include oxidation of fats and oils, hydrolysis of fats and proteins, enzymatic browning, and microbial growth. Each of these undesired processes will occur at lower rates when the food is stored in a 5°C refrigerator instead of a 25°C cabinet. The 20°C decrease in temperature would cause the rates of these reactions to be about four times lower. Food preservation through the use of refrigerators is another example of Chemistry for Better Living.
Surface Area
 In 2008, a fatal explosion occurred at the Imperial Sugar Refinery plant just outside of Savannah, Georgia. The accident represents one of the deadliest industrial dust explosion accidents in history. Imperial Sugar was one of the largest producers of granulated sugar and specialty sugar products. In the plant, there was an abundance of fine sugar dust buildup on surfaces and floors from years of transport of the product by conveyor belts from the production line to the storage silos. These conditions were inviting an accident. On February 7, a spark from an overheated bearing on a conveyer line set some nearby sugar dust on fire. The rapid buildup of heat set off a chain reaction of explosions in nearby parts of the factory that harbored piles of sugar dust. Fireballs in one part of the factory set off explosions in other parts of the factory and in very little time, the entire factory was destroyed in seconds.
In 2008, a fatal explosion occurred at the Imperial Sugar Refinery plant just outside of Savannah, Georgia. The accident represents one of the deadliest industrial dust explosion accidents in history. Imperial Sugar was one of the largest producers of granulated sugar and specialty sugar products. In the plant, there was an abundance of fine sugar dust buildup on surfaces and floors from years of transport of the product by conveyor belts from the production line to the storage silos. These conditions were inviting an accident. On February 7, a spark from an overheated bearing on a conveyer line set some nearby sugar dust on fire. The rapid buildup of heat set off a chain reaction of explosions in nearby parts of the factory that harbored piles of sugar dust. Fireballs in one part of the factory set off explosions in other parts of the factory and in very little time, the entire factory was destroyed in seconds.
(View the U.S. Chemical Safety Board’s video report on the Imperial Sugar Refinery explosion.)
Sugar is combustible and the rate of combustion is dependent upon the size of the particle. Reaction rates are greater for smaller and finer-sized particles. Sugar dust will react faster than sugar grains which will react faster than a sugar cube. The same can be said of any solid-state reactant and any reaction. Powdered reactants react faster than larger grains and massive clumps of reactant materials. We attribute this to the amount of exposure to the surface of the reactants. A fine dust particle will expose more surface area to other reactants and increase the rate of the chemical reaction. The general rule is that an increase in the surface area of a solid reactant will increase the reaction rate. Surface area is the third variable that affects the rate of a reaction.
Calcium carbonate (chalk) reacts with acids like the acetic acid in vinegar to produce carbon dioxide gas. The rate at which the reaction occurs can be gauged by observing the rate at which the carbon dioxide bubbles are produced. The chemical equation is
CaCO3(s) + 2 HC2H3O2(aq) → Ca(C2H3O2)2(aq) + H2O(l) + CO2(g)
Let’s suppose the following experiment is performed. Three small, identical pieces of chalk (CaCO3) are obtained. One of them is left untouched. One is broken into several smaller pieces using a hammer. And the third is ground up into a fine powder using a mortar and pestle. The three samples of CaCO3 are added to the same amount of vinegar in a beaker. What would we observe?
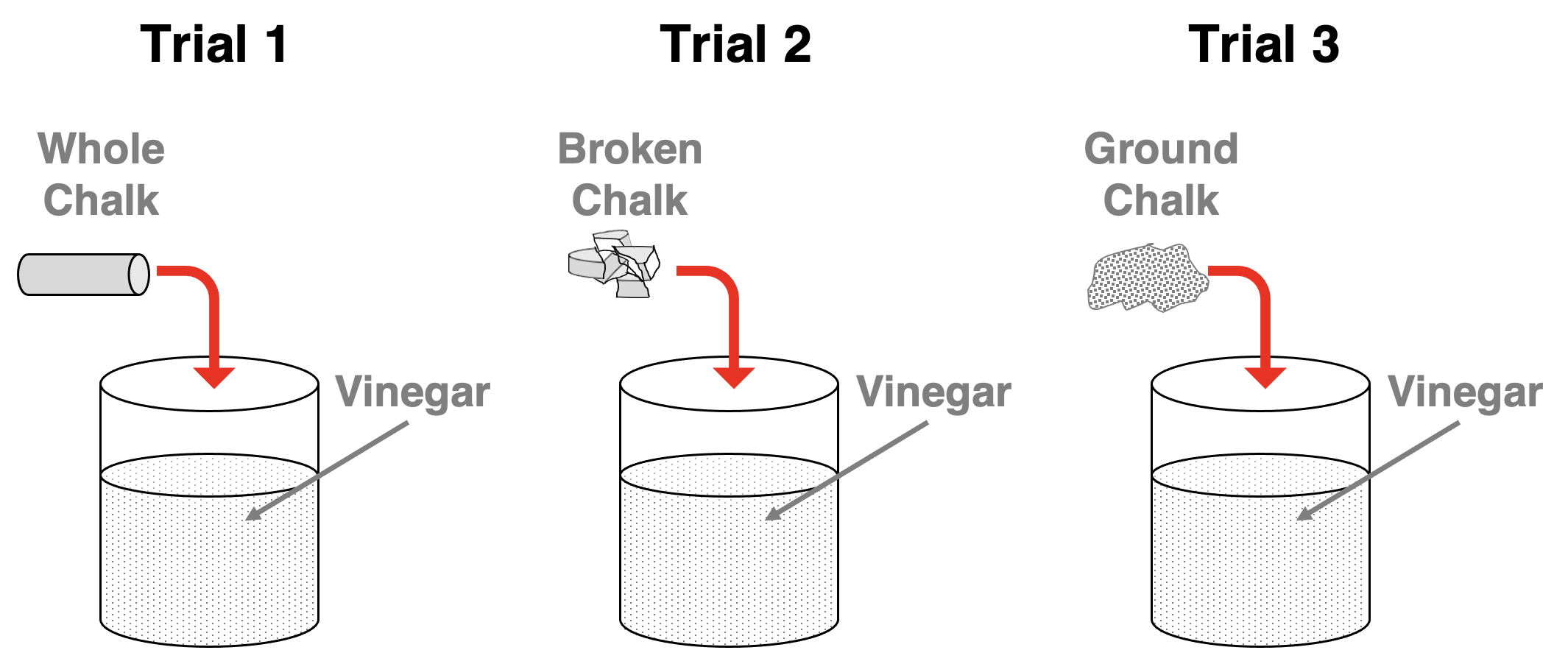
 Since reaction rates increase with increasing surface area, we would observe the indicators of the greatest rate for Trial 3 and the lowest rate for Trial 1. We would observe that during the early stages of the reaction, the bubbles of CO2 gas are most vigorously generated in Trial 3 and least vigorously generated in Trial 1. The reaction between the CaCO3 and the HC2H3O2 would occur faster when the chalk is broken down into dust-size partciles. The observations of the CO2 gas bubbles confirms the claim that reaction rates increase with increasing surface area of a solid reactant.
Since reaction rates increase with increasing surface area, we would observe the indicators of the greatest rate for Trial 3 and the lowest rate for Trial 1. We would observe that during the early stages of the reaction, the bubbles of CO2 gas are most vigorously generated in Trial 3 and least vigorously generated in Trial 1. The reaction between the CaCO3 and the HC2H3O2 would occur faster when the chalk is broken down into dust-size partciles. The observations of the CO2 gas bubbles confirms the claim that reaction rates increase with increasing surface area of a solid reactant.
Use of a Catalyst
The fourth means of altering the rate of a reaction involves the use of a catalyst. A catalyst is a substance that increases the rate of a reaction without itself undergoing any permanent change. Since a catalyst does not undergo a permanent change, it is not included as a reactant in the chemical reaction.
 Hydrogen peroxide (H2O2) is a strong oxidizing agent. In its pure form, it is and abnormally dangerous and unstable liquid. It is often diluted with water and available for purchase in stores as a more stable 3% aqueous solution. Hydrogen peroxide naturally decomposes into water and oxygen gas.
Hydrogen peroxide (H2O2) is a strong oxidizing agent. In its pure form, it is and abnormally dangerous and unstable liquid. It is often diluted with water and available for purchase in stores as a more stable 3% aqueous solution. Hydrogen peroxide naturally decomposes into water and oxygen gas.
2 H2O2(aq) → 2 H2O(l) + O2(g)
Under normal conditions, its decomposition rate is extremely slow. A quart-sized of 3% H2O2(aq) kept in its original brown bottle at room temperature or below will remain effective for as long as 3 years. Obviously, that is a long shelf life, indicating a rather slow decomposition reaction.
The rate of decomposition of H2O2 can be increased through the use of a catalyst. A once common homespun strategy for cleaning a wound involved the use of H2O2 as a disinfectant. A small amount of 3% H2O2 was poured into the wound. Its decomposition rate would be increased by the presence of an enzyme naturally present in body tissues, aptly named catalase. The catalase would act as a catalyst to speed up the decomposition of H2O2. The H2O2 would kill bacteria and the oxygen being produced by its decomposition would lift contaminants and debris from the wound. The practice is no longer recommended since the H2O2 also harms healthy tissue and impedes the healing process.
 One set of commercial products that relies on the catalytic decomposition of H2O2 are contact lens cleaners. One of the more popular of these products is named Clear Care Plus. A small vial is filled with the contact lens cleaner, consisting of 3% H2O2. The lid of the vial is equipped with two receptacles that hold the contact lenses. The magic happens below the lens holders. A platinum catalyst is attached to the lid assembly. The contact lens cleaner will do nothing until it is contact with the catalyst. Once the lid is secured and the catalyst is immersed in the H2O2, the decomposition begins at a rapid rate. The H2O2 kills bacteria and breaks up protein deposits to disinfect the lens. Meanwhile, the bubbling action of the oxygen gas that is produced by its decomposition helps to remove the debris from the lens. Because catalysts do not become used up in a reaction, the catalyst will last forever … or at least until you purchase your next bottle. Those contact lens wearers who depend upon this H2O2-based contact disinfection system will readily tell you that it is a great example of Chemistry for Better Living.
One set of commercial products that relies on the catalytic decomposition of H2O2 are contact lens cleaners. One of the more popular of these products is named Clear Care Plus. A small vial is filled with the contact lens cleaner, consisting of 3% H2O2. The lid of the vial is equipped with two receptacles that hold the contact lenses. The magic happens below the lens holders. A platinum catalyst is attached to the lid assembly. The contact lens cleaner will do nothing until it is contact with the catalyst. Once the lid is secured and the catalyst is immersed in the H2O2, the decomposition begins at a rapid rate. The H2O2 kills bacteria and breaks up protein deposits to disinfect the lens. Meanwhile, the bubbling action of the oxygen gas that is produced by its decomposition helps to remove the debris from the lens. Because catalysts do not become used up in a reaction, the catalyst will last forever … or at least until you purchase your next bottle. Those contact lens wearers who depend upon this H2O2-based contact disinfection system will readily tell you that it is a great example of Chemistry for Better Living.

Perhaps the most popular example of the catalytic decomposition of H2O2 is a popular chemistry demonstration sometimes referred to as the elephant toothpaste demonstration. A catalyst is added to a flask of H2O2, dishwashing soap, and food coloring (for added pizzazz). In chemistry classrooms, potassium iodide (KI) is a commonly used catalyst, but yeast (which contains catalase) works just as well. The catalyst increases the reaction rate, producing oxygen gas at a rapid rate. The oxygen bubbles through the dishwashing soap to produce a foamy mixture. The decomposition reaction is exothermic. The heat that is produced contributes to the effect, causing the foam to rapidly expand and be projected out the mouth of the flask. The foamy product looks like toothpaste squeezed out of a tube … enough for an elephant.
To see it in action, watch Mark Rober’s World’s Largest Elephant Toothpaste Experiment.
We have now identified the main variables that affect the rate of a reaction. But we haven’t explained why. In terms of particles, why does concentration, temperature, surface area, and the use of a catalyst affect the reaction rate? On our next page of Lesson 1, we will present a particle model known as the Collision Model to explain the underlying reasons for these variable relationships. Before you click forward to learn more, take some time to make sure you to ensure you understand Lesson 1b by using one or more of the suggestions in the Before You Leave section.
Before You Leave
- Download our Study Card on Factors Affecting Reaction Rates. Save it to a safe location and use it as a review tool. (Coming soon.)
- Try our Concept Builder titled Reaction Rates and the Collision Model. The first activity (of three activities) is a great follow-up to this lesson.
- Try our Science Reasoning Activity titled Reaction Rates. The first and second activities (of four activities) provide a great follow-up to this Lesson.
- The Check Your Understanding section below include questions with answers and explanations. It provides a great chance to self-assess your understanding.
Check Your Understanding
Use the following questions to assess your understanding. Tap the Check Answer buttons when ready.
1. Three trials of a reaction are performed in a beaker. The reaction is exothermic. The concentration of one of the reactants is varied systematically from trial to trial. Ten seconds into each trial, a thermometer is used to measure the temperature of the beaker. Here’s the results:
Trial 1: T = 38°C
Trial 2: T = 52°C
Trial 3: T = 28°C
Rank the three trials in increasing order of reactant concentration, from lowest to highest concentration.
2. Three trials of a reaction are performed in a beaker. The reaction is endothermic. The concentration of one of the reactants is varied systematically from trial to trial. Ten seconds into each trial, a thermometer is used to measure the temperature of the beaker. Here’s the results:
Trial 1: T = 12°C
Trial 2: T = 23°C
Trial 3: T = 19°C
Rank the three trials in increasing order of reactant concentration, from lowest to highest concentration.
3. Three trials of a reaction involving a solid and an aqueous solution are performed in a flask. The temperature of the aqueous solution is varied from trial to trial. The reaction results in the production of a red-colored product. Observations are made of the intensity of the red color at the 10-second mark. Here’s the results:
Trial 1: No red color observed
Trial 2: Intense red color observed
Trial 3: Pale red color observed
Rank the three trials in increasing order of temperature from lowest to highest temperature.
4. A student lab group is assigned the task of determining how the surface area of a Alka Seltzer tablet would affect the reaction rate.
- Describe a procedure for conducting such a study.
- Identify the types of observations could be made as evidence in support of a conclusion.
- Explain how the observations could be interpretted to make and support a conclusion.
5. Do catalysts ever slow down a reaction? Or can they only speed up a reaction?