Quantum Mechanics - Questions
The Quantum Mechanics Concept Builder consists of 32 Questions organized into 12 Question Groups and spread across three activities. The first activity is a
Matching Pairs activity in which learner are presented 8 terms or statements on a grid and must match the terms or statements with one another. There are two sets of Matching Pair grids. The Concept Builder scrambles the arrangement of ideas within the Matching Pairs grid. Otherwise, all students will have the same set of terms. The second activity is called
Law Breakers. Learners are presented with three sets of quantum numbers and must identify any set that violates the rules for allowed quantum number values. The third activity is titled
Two Truths and One Lie and presents three statements regarding atomic orbitals. Learners must identify the statement that is false. For the second and third activity, students are selected a question at random from each Question Group. The order in which the Question Group is presented to the student is also randomzied. It is unlikely that two side-by-side students will have the same experience.
Teachers are encouraged to view the questions in order to judge which activities are most appropriate for their classes.
The Physics Classroom grants teachers and other users the right to print these questions for private use. Users are also granted the right to copy the text and modify it for their own use. However, this document should not be uploaded to other servers for distribution to and/or display by others. The Physics Classroom website should remain the only website or server from which the document is distributed or displayed. We also provide a PDF that teachers can use under the same conditions. We have included a link to the PDF near the bottom of this page.
Quantum Mechanics
Activity 1: Matching Pairs: Quantum Numbers
This activity presents learners with 8 different statements that must be matched by meaning. Learners tap on the statements to select them and then tap on the Check Match button. The order of the statements is randomized. A mis-matched pair restarts the
game and re-randomizes the order of the statements. The statements are ...
Question Group 1:
Question 1:
Describes the shape of the orbitals.
Quantum number
n
Quantum number
ml
Describes the spatial orientation of orbitals.
Quantum number
ms
Describes the energy of the electron shell.
Describes the direction which an electron spins.
Quantum number
l
Question Group 2:
Question 2:
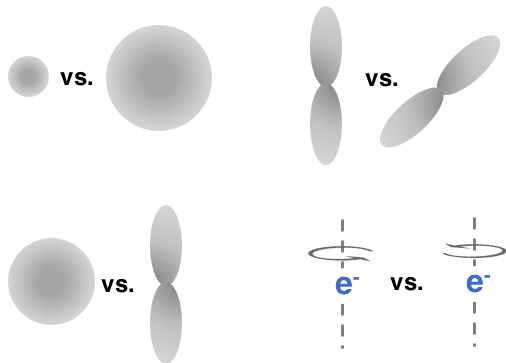
n = 1 versus
n = 2
l = 0 versus
l = 1
ml = 0 versus
ml = 1
ms = ½ versus
ms = -½
 Activity 2: Law Breakers
Question Group 3
Question 3
Activity 2: Law Breakers
Question Group 3
Question 3
Consider the set of quantum number values shown below. Identify any set that represents non-allowed quantum numbers. Select all that apply.
Question 4
Consider the set of quantum number values shown below. Identify any set that represents non-allowed quantum numbers. Select all that apply.
Question 5
Consider the set of quantum number values shown below. Identify any set that represents non-allowed quantum numbers. Select all that apply.
Question Group 4
Question 6
Consider the set of quantum number values shown below. Identify any set that represents non-allowed quantum numbers. Select all that apply.
Question 7
Consider the set of quantum number values shown below. Identify any set that represents non-allowed quantum numbers. Select all that apply.
Question 8
Consider the set of quantum number values shown below. Identify any set that represents non-allowed quantum numbers. Select all that apply.
Question Group 5
Question 9
Consider the set of quantum number values shown below. Identify any set that represents non-allowed quantum numbers. Select all that apply.
Question 10
Consider the set of quantum number values shown below. Identify any set that represents non-allowed quantum numbers. Select all that apply.
Question 11
Consider the set of quantum number values shown below. Identify any set that represents non-allowed quantum numbers. Select all that apply.
Question Group 6
Question 12
Consider the set of quantum number values shown below. Identify any set that represents non-allowed quantum numbers. Select all that apply.
Question 13
Consider the set of quantum number values shown below. Identify any set that represents non-allowed quantum numbers. Select all that apply.
Question 14
Consider the set of quantum number values shown below. Identify any set that represents non-allowed quantum numbers. Select all that apply.
Question Group 7
Question 15
Consider the set of quantum number values shown below. Identify any set that represents non-allowed quantum numbers. Select all that apply.
Question 16
Consider the set of quantum number values shown below. Identify any set that represents non-allowed quantum numbers. Select all that apply.
Question 17
Consider the set of quantum number values shown below. Identify any set that represents non-allowed quantum numbers. Select all that apply.
Question Group 8
Question 18
Consider the set of quantum number values shown below. Identify any set that represents non-allowed quantum numbers. Select all that apply.
Question 19
Consider the set of quantum number values shown below. Identify any set that represents non-allowed quantum numbers. Select all that apply.
Question 20
Consider the set of quantum number values shown below. Identify any set that represents non-allowed quantum numbers. Select all that apply.
Activity 3: Two Truths and a Lie
Question Group 9
Question 21
Consider the three statements. Two are true. One is false. Select the false statement.
When all the d orbitals at a given energy level are full, there a total of 10 electrons in those orbitals.
A larger orbital has the capacity to hold more electrons than a smaller orbital.
There are four different types of orbitals in the fourth principal energy level (n = 4).
Question 22
Consider the three statements. Two are true. One is false. Select the false statement.
Every single p orbital can hold a maximum of six electrons.
There are four different types of orbitals in the fourth principal energy level (n = 4).
The second principal energy level (n=2) does not have any d orbitals.
Question 23
Consider the three statements. Two are true. One is false. Select the false statement.
For any given energy level, the maximum number of electrons present in f orbitals is 14.
The second principal energy level (n=2) does not have any d orbitals.
A larger orbital has the capacity to hold more electrons than a smaller orbital.
Question Group 10
Question 24
Consider the three statements. Two are true. One is false. Select the false statement.
For any given energy level, the maximum number of electrons present in f orbitals is 14.
There are two different types of orbitals in the second principal energy level (n = 2).
For any given energy level, the maximum number of electrons present in p orbitals is 10.
Question 25
Consider the three statements. Two are true. One is false. Select the false statement.
When all the p orbitals at a given energy level are full, there a total of six electrons in those orbitals.
For any given energy level, the maximum number of electrons present in d orbitals is 14.
There are three different types of orbitals in the third principal energy level (n = 3).
Question 26
Consider the three statements. Two are true. One is false. Select the false statement.
For any given energy level, the maximum number of electrons present in p orbitals is 6.
There are five different d orbitals in the fourth principal energy level (n=3)
For any given energy level, the maximum number of electrons present in f orbitals is 10.
Question Group 11
Question 27
Consider the three statements. Two are true. One is false. Select the false statement.
There are three different p orbitals in the fourth principal energy level (n=4)
The fourth principal energy level (n=4) has more d orbitals than the third principal energy level (n=3).
When all the f orbitals at a given energy level are full, there a total of 14 electrons in those orbitals.
Question 28
Consider the three statements. Two are true. One is false. Select the false statement.
Every single d orbital can hold a maximum of two electrons.
There are three different p orbitals in the fourth principal energy level (n=4)
The third principal energy level (n=3) does not have any d orbitals.
Question 29
Consider the three statements. Two are true. One is false. Select the false statement.
The third principal energy level (n=3) has more p orbitals than the second principal energy level (n=2).
When all the d orbitals at a given energy level are full, there a total of 10 electrons in those orbitals.
The third principal energy level (n=3) has the capacity to hold up to 18 electrons.
Question Group 12
Question 30
Consider the three statements. Two are true. One is false. Select the false statement.
There are 4 different d orbitals in the fourth principal energy level (n=4)
The second principal energy level (n=2) has the capacity to hold up to 8 electrons.
Every single p orbital can hold a maximum of two electrons.
Question 31
Consider the three statements. Two are true. One is false. Select the false statement.
The third principal energy level (n=3) has the capacity to hold up to 18 electrons.
There are 10 different d orbitals in the fifth principal energy level (n=5)
There are four different types of orbitals in the fourth principal energy level (n = 4).
Question 32
Consider the three statements. Two are true. One is false. Select the false statement.
There are four different types of orbitals in the fourth principal energy level (n = 4).
When all the f orbitals at a given energy level are full, there a total of 14 electrons in those orbitals.
There are 10 different d orbitals in the third principal energy level (n=3)