Hold down the T key for 3 seconds to activate the audio accessibility mode, at which point you can click the K key to pause and resume audio. Useful for the Check Your Understanding and See Answers.
Lesson 2: Classifying Chemical Reactions
Part b: Combustion Reactions
Part a:
Decomposition and Synthesis Reactions
Part b: Combustion Reactions
Part c:
Single Replacement Reactions
Part d:
Double Replacement Reactions
Part e:
Predicting Products
 Reaction Types and Predicting Products
Reaction Types and Predicting Products
Lesson 2 began with a discussion of reaction types. The emphasis was placed upon using knowledge of reaction types to predict the products of a reaction. Five reaction types were identified. In Lesson 2a, synthesis (a.k.a., combination) and decomposition reactions were discussed. Several examples were used to demonstrate how a knowledge of these two types of reactions could be used to predict products and to construct balanced chemical equations. In Lesson 2b, the theme of predicting products and writing balanced chemical equations will be expanded to a third type of reactions – combustion reactions.
What is a Combustion Reaction?
A combustion reaction is a reaction of a substance with oxygen gas to release relatively large amounts of energy. Because combustion reactions are often characterized by the presence of flames, a substance undergoing combustion is often said to be burning.
The substance that reacts with oxygen can be an element or a compound. The combustion of an element results in the formation of the oxide of that element. Both metal and nonmetal elements can undergo combustion. For instance, the metal magnesium (Mg) reacts with oxygen to produce the oxide of magnesium – MgO. The balanced chemical equation is …
2 Mg(s) + O2(g) → 2 MgO(s)
Sulfur is a nonmetal that undergoes combustion. The products of nonmetal combustion are less predictable. While the product is always an oxide of the nonmetal, the formula is not always predictable since there are often more than one possible nonmetal oxides that can be produced. Sulfur most commonly forms SO2(g) when undergoing combustion.
S(s) + O2(g) → SO2(g)
It is worth noting that both reactions above can be classified as synthesis reactions. It is not uncommon for a reaction to fit into more than one category.
 Hydrocarbon Combustion
Hydrocarbon Combustion
Compounds can also undergo combustion. A class of compounds known as hydrocarbons is notorious for undergoing combustion. Hydrocarbons, as the name implies, are compounds containing the elements hydrogen and carbon. Some commonly burned hydrocarbons include methane (CH4), propane (C3H8), butane (C4H10), and octane (C8H18). Methane is the main component of natural gas. Natural gas is the most common fuel used for cooking and heating of homes (and for Bunsen burners in chemistry labs). Propane is the fuel used for outdoor gas grills. Butane is the fuel used in handheld lighters. And octane is the primary component in the gasoline used to fuel automobiles (as of this writing). The value of these hydrocarbons lies in the large amount of energy produced when they undergo combustion.
As mentioned, the products of combustion are the oxides of the substance that is being burned. When a hydrocarbon reacts with oxygen, the products are carbon dioxide gas and water vapor. These are the oxides of carbon and hydrogen. The balanced chemical equation for the combustion of methane is …
CH4(g) + 2 O2(g) → CO2(g) + 2 H2O(g)
The balanced chemical equation for the combustion of propane is …
C3H8(g) + 5 O2(g) → 3 CO2(g) + 4 H2O(g)
 There is undeniable value in hydrocarbon combustion. The bond breaking, rearrangement of atoms, and bond forming process results in the release of relatively large quantities of energy. Both products are non-toxic. Unfortunately, there is a big HOWEVER. Both products are greenhouse gases. Most concerning is the CO2, which prevents heat irradiated from Earth from exiting our atmosphere. Carbon dioxide is the main contributor to global climate change. The problem of global climate change exists due to the ever-increasing dependence of humankind upon hydrocarbon combustion. The plot below displays the slow and steady increase in global temperatures over the past century. These increasing global temperatures can be correlated to numerous negative impacts such as rising sea levels and potential displacement of coastal communities, changing agricultural zones, the disruption of wildlife habitats, and extreme weather conditions (heatwaves, droughts, intense storms, etc.).
There is undeniable value in hydrocarbon combustion. The bond breaking, rearrangement of atoms, and bond forming process results in the release of relatively large quantities of energy. Both products are non-toxic. Unfortunately, there is a big HOWEVER. Both products are greenhouse gases. Most concerning is the CO2, which prevents heat irradiated from Earth from exiting our atmosphere. Carbon dioxide is the main contributor to global climate change. The problem of global climate change exists due to the ever-increasing dependence of humankind upon hydrocarbon combustion. The plot below displays the slow and steady increase in global temperatures over the past century. These increasing global temperatures can be correlated to numerous negative impacts such as rising sea levels and potential displacement of coastal communities, changing agricultural zones, the disruption of wildlife habitats, and extreme weather conditions (heatwaves, droughts, intense storms, etc.).
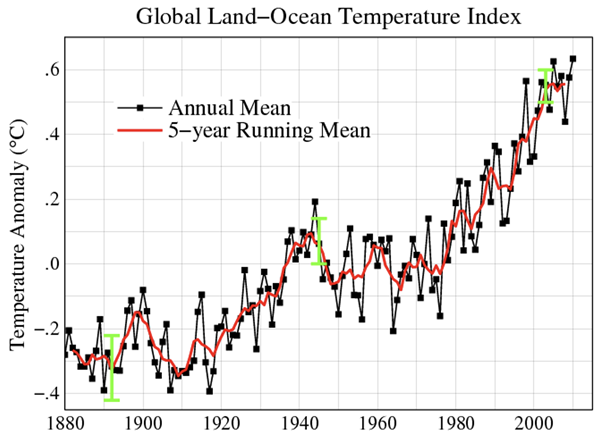
Image: Public Domain
Writing Balanced Equations for Combustion Reactions
 A common task required in introductory Chemistry courses is the writing of balanced chemical equations for combustion reactions. The first step is to identify the reactants the products. Reactants include the substance undergoing combustion and oxygen gas. The products are the oxides of the elements in the substance undergoing combustion. The second step involves writing a skeleton equation for the combustion reaction. The third step involves adding coefficients to balance the chemical equation. The third step may result in a half-number coefficient, in which case all coefficients will need to be multiplied by two in order to acquire the more acceptable whole number coefficients.
A common task required in introductory Chemistry courses is the writing of balanced chemical equations for combustion reactions. The first step is to identify the reactants the products. Reactants include the substance undergoing combustion and oxygen gas. The products are the oxides of the elements in the substance undergoing combustion. The second step involves writing a skeleton equation for the combustion reaction. The third step involves adding coefficients to balance the chemical equation. The third step may result in a half-number coefficient, in which case all coefficients will need to be multiplied by two in order to acquire the more acceptable whole number coefficients.
We offer several examples below. We recommend that students try to complete each example on their own and then use the View Answer button to check the answer and/or complete solution. There are additional practice ideas provided in the Before You Leave section. Balancing equations is not a great spectator sport. It is important to do more than watch someone else balance an equation.
Example 1
Internal combustion engines of automobiles mix octane gasoline with oxygen and provide a spark to combust the octane (C8H18). The energy released is used to move pistons and turn wheel axles to power the automobile. Write the balanced chemical equation for the combustion of octane.
 Example 2
Example 2
Canned heat, made popular by Sterno, is usually composed of methanol, CH4O. Write the balanced chemical equation for the combustion of methanol.
Example 3
Kerosene is a mixture of a variety of compounds containing between 12 and 15 carbons. The most burned component of the mixture is C12H26. Write the balanced chemical equation for the combustion of C12H26.
Example 4
Write the balanced chemical equation for the combustion of calcium.
Example 5
Liquid hydrazine, N2H4(l), is a commonly used rocket fuel. When burned in an oxygen-rich environment, nitrogen dioxide and water vapor are produced. Write the balanced chemical equation for the combustion of hydrazine.
Before You Leave
- Download our Study Card on Types of Reactions. (It covers Lessons 2a, 2b, 2c, and some of Lesson 2d.) Save it to a safe location and use it as a review tool.
- Once you have some comfort with Lessons 2a through 2d, try our Chemical Reaction Type Concept Builder. It will provide awesome practice on all five reaction types.
- The Check Your Understanding section below includes questions with answers and explanations. It provides a great chance to self-assess your understanding.
Check Your Understanding
Use the following questions to assess your understanding. Tap the Check Answer buttons when ready.
1. Explain how to tell if a reaction is a combustion reaction.
2. Describe how to predict the products of a combustion reaction.
3. Write the balanced chemical equation for the combustion of cyclohexane (C
6H
12).
4. Write the balanced chemical equation for the combustion of paraffin (C
25H
52).
5. Write the balanced chemical equation for the combustion of ethanol (C
2H
6O).