Line Spectra - Questions
The Line Spectra Concept Builder is comprised of 22 questions. The questions are divided into 7 different Question Groups and spread across three different activities. Questions in the same group are rather similar to one another. The Concept Builder is coded to select at random a question from each group until a student is successful with that group of questions.
There are three activities in the Concept Builder. Those three activities are differentiated as follows:
- Paragraph Completion: Question Group 1 ... Complete a paragraph by determining the appropriate phrase for each of 7 blanks.
- Line Identification: Question Groups 2 - 4 ... Identify the color associated with each of three lines on an emission spectrum if given their relative wavelength, frequency, or photon energy.
- Match That Color: Question Groups 5 - 7 ... Associate one of three given colors with three given electron transitions on a given energy level diagram.
The questions from each group are shown below. Teachers are encouraged to view the questions in order to judge which difficulty levels are most appropriate for their classes. We recommend providing students two or more options.
The Physics Classroom grants teachers and other users the right to print these questions for private use. Users are also granted the right to copy the text and modify it for their own use. However, this document should not be uploaded to other servers for distribution to and/or display by others. The Physics Classroom website should remain the only website or server from which the document is distributed or displayed. We also provide a PDF that teachers can use under the same conditions. We have included a link to the PDF near the bottom of this page.
Line Spectra Questions
Activity 1 – Pargraph Completion
Question Group 1
Question 1
There is only one question in this Activity - a paragraph completion question with seven blanks to fill in. The paragraph is ...

Options for blanks include:
1. a continuous range of , only certain quantized
2. heat or electricity is applied, the object is touched or rubbed, a proton or neutron is added
3. releases , absorbs
4. a resting state, a ground state, an excited state, a united state
5. a phase change, a chemical reaction, the release of a photon
6. energy of the photon, enthalpy change of the reaction, heat of vaporization or fusion
7. a continuous rainbow of color, lines of a specific color, a magnificent fireworks display
Activity 2: Match That Color
Question Group 2
Question 2
Consider three colored lines on a line spectra - red, orange, and violet. Wavelengths associated with the lines are shown below. Match the wavelengths to the color of the lines.

Question 3
Consider three colored lines on a line spectra - blue, green, and violet. Wavelengths associated with the lines are shown below. Match the wavelengths to the color of the lines.

Question 4
Consider three colored lines on a line spectra - red, yellow, and green. Wavelengths associated with the lines are shown below. Match the wavelengths to the color of the lines.

Question Group 3
Question 5
Consider three colored lines on a line spectra - violet, red, and green. Frequencies associated with the lines are shown below. Match the frequencies to the color of the lines.

Question 6
Consider three colored lines on a line spectra - blue, green, and vioilet. Frequencies associated with the lines are shown below. Match the frequencies to the color of the lines.

Question 7
Consider three colored lines on a line spectra - red, orange, and green. Frequencies associated with the lines are shown below. Match the frequencies to the color of the lines.

Question Group 4
Question 8
Consider three colored lines on a line spectra - blue, green, and red. Photon energy values associated with the lines are shown below. Match the energy values to the color of the lines.

Question 9
Consider three colored lines on a line spectra - violet, yellow, and orange. Photon energy values associated with the lines are shown below. Match the energy values to the color of the lines.

Question 10
Consider three colored lines on a line spectra - red, orange, and yellow. Photon energy values associated with the lines are shown below. Match the energy values to the color of the lines.

Activity 3: Match That Color
Question Group 5
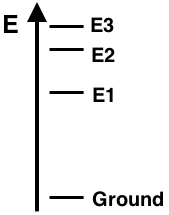 Question 11
Question 11
The energy level diagram for Element X shows the ground state and excited states E1, E2, and E3. The line spectra for this element displays three visible emission lines - violet, green, and red. The lines correspond to the electron transitions indicated below. Based on the properly-scaled energy levels shown in the diagram, match the three colors to the corresponding transitions.
e-Transition from E3 to E1: ___________
e-Transition from E2 to E1: ___________
e-Transition from E1 to Ground: ___________
Finally, the transition from E3 to Ground state would not be visible since it would correspond to the emission of ...
 Question 12
Question 12
The energy level diagram for Element X shows the ground state and excited states E1, E2, and E3. The line spectra for this element displays three visible emission lines - violet, green, and red. The lines correspond to the electron transitions indicated below. Based on the properly-scaled energy levels shown in the diagram, match the three colors to the corresponding transitions.
e-Transition from E3 to E3: ___________
e-Transition from E3 to E1: ___________
e-Transition from E2 to E1: ___________
Finally, the transition from E1 to Ground state would not be visible since it would correspond to the emission of ...
 Question 13
Question 13
The energy level diagram for Element X shows the ground state and excited states E1, E2, and E3. The line spectra for this element displays three visible emission lines - violet, green, and orange. The lines correspond to the electron transitions indicated below. Based on the properly-scaled energy levels shown in the diagram, match the three colors to the corresponding transitions.
e-Transition from E3 to E1: ___________
e-Transition from E2 to E1: ___________
e-Transition from E1 to Ground: ___________
Finally, the transition from E3 to E2 would not be visible since it would correspond to the emission of ...
 Question 14
Question 14
The energy level diagram for Element X shows the ground state and excited states E1, E2, and E3. The line spectra for this element displays three visible emission lines - blue, yellow, and red. The lines correspond to the electron transitions indicated below. Based on the properly-scaled energy levels shown in the diagram, match the three colors to the corresponding transitions.
e-Transition from E3 to E1: ___________
e-Transition from E2 to E1: ___________
e-Transition from E1 to Ground: ___________
Finally, the transition from E3 to E2 would not be visible since it would correspond to the emission of ...
Question Group 6
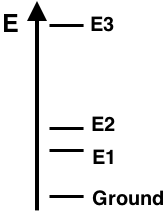 Question 15
Question 15
The energy level diagram for Element X shows the ground state and excited states E1, E2, and E3. The line spectra for this element displays three visible emission lines - violet, green, and red. The lines correspond to the electron transitions indicated below. Based on the properly-scaled energy levels shown in the diagram, match the three colors to the corresponding transitions.
e-Transition from E3 to E2: ___________
e-Transition from E2 to Ground: ___________
e-Transition from E1 to Ground: ___________
Finally, the transition from E3 to Ground state would not be visible since it would correspond to the emission of ...
 Question 16
Question 16
The energy level diagram for Element X shows the ground state and excited states E1, E2, and E3. The line spectra for this element displays three visible emission lines - violet, blue, and orange. The lines correspond to the electron transitions indicated below. Based on the properly-scaled energy levels shown in the diagram, match the three colors to the corresponding transitions.
e-Transition from E3 to E2: ___________
e-Transition from E2 to Ground: ___________
e-Transition from E1 to Ground: ___________
Finally, the transition from E2 to E1 would not be visible since it would correspond to the emission of ...
 Question 17
Question 17
The energy level diagram for Element X shows the ground state and excited states E1, E2, and E3. The line spectra for this element displays three visible emission lines - violet, green, and red. The lines correspond to the electron transitions indicated below. Based on the properly-scaled energy levels shown in the diagram, match the three colors to the corresponding transitions.
e-Transition from E3 to E2: ___________
e-Transition from E2 to E1: ___________
e-Transition from E2 to Ground: ___________
Finally, the transition from E3 to Ground state would not be visible since it would correspond to the emission of ...
 Question 18
Question 18
The energy level diagram for Element X shows the ground state and excited states E1, E2, and E3. The line spectra for this element displays three visible emission lines - blue, yellow, and red. The lines correspond to the electron transitions indicated below. Based on the properly-scaled energy levels shown in the diagram, match the three colors to the corresponding transitions.
e-Transition from E3 to E2: ___________
e-Transition from E2 to E1: ___________
e-Transition from E2 to Ground: ___________
Finally, the transition from E2 to E1 would not be visible since it would correspond to the emission of ...
Question Group 7
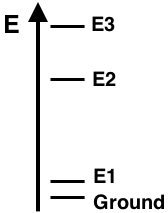 Question 19
Question 19
The energy level diagram for Element X shows the ground state and excited states E1, E2, and E3. The line spectra for this element displays three visible emission lines - violet, green, and red. The lines correspond to the electron transitions indicated below. Based on the properly-scaled energy levels shown in the diagram, match the three colors to the corresponding transitions.
e-Transition from E3 to E2: ___________
e-Transition from E2 to E1: ___________
e-Transition from E2 to Ground: ___________
Finally, the transition from E3 to Ground state would not be visible since it would correspond to the emission of ...
 Question 20
Question 20
The energy level diagram for Element X shows the ground state and excited states E1, E2, and E3. The line spectra for this element displays three visible emission lines - violet, blue, and orange. The lines correspond to the electron transitions indicated below. Based on the properly-scaled energy levels shown in the diagram, match the three colors to the corresponding transitions.
e-Transition from E3 to E2: ___________
e-Transition from E2 to E1: ___________
e-Transition from E2 to Ground: ___________
Finally, the transition from E1 to Ground state would not be visible since it would correspond to the emission of ...
 Question 21
Question 21
The energy level diagram for Element X shows the ground state and excited states E1, E2, and E3. The line spectra for this element displays three visible emission lines - violet, green, and red. The lines correspond to the electron transitions indicated below. Based on the properly-scaled energy levels shown in the diagram, match the three colors to the corresponding transitions.
e-Transition from E3 to E2: ___________
e-Transition from E2 to E1: ___________
e-Transition from E1 to Ground: ___________
Finally, the transition from E3 to Ground state would not be visible since it would correspond to the emission of ...
 Question 22
Question 22
The energy level diagram for Element X shows the ground state and excited states E1, E2, and E3. The line spectra for this element displays three visible emission lines - violet, blue, and red. The lines correspond to the electron transitions indicated below. Based on the properly-scaled energy levels shown in the diagram, match the three colors to the corresponding transitions.
e-Transition from E3 to E2: ___________
e-Transition from E2 to E1: ___________
e-Transition from E1 to Ground: ___________
Finally, the transition from E2 to Ground state would not be visible since it would correspond to the emission of ...