Molecular Polarity - Questions
The Molecular Polarity Concept Builder is comprised of 54 questions organized into 18 Question Groups and spread across three different difficulty levels. The
Apprentice Difficulty Level includes 6 Question Groups and 18 total questions. Each question presents information about three molecules and students must decide if each molecule is polar or non-polar. The presented information includes the AXE notation, the molecular geometry, and a ball-and-stick model. The
Master Difficulty Level includes 6 Question Groups and 18 total questions. Each question provides a chemical formula and Lewis electron dot structure for a molecule. Students must determine the molecular geometry from the electron dot structure and then determine if the molecule is polar or non-polar. All structures are limited to a maximum of four electron groups around the central atom. The
Wizard Difficulty Level is similar to the Master Level with the exception that all structures have five or six electron groups around the central atom. Students must still determine the molecular geometry and the polarity of the molecule.
The Physics Classroom grants teachers and other users the right to print these questions for private use. Users are also granted the right to copy the text and modify it for their own use. However, this document should not be uploaded to other servers for distribution to and/or display by others. The Physics Classroom website should remain the only website or server from which the document is distributed or displayed. We also provide a PDF that teachers can use under the same conditions. We have included a link to the PDF near the bottom of this page.
Molecular Polarity
Questions 1 -18 Image Credits: Wikimedia Commons (Public Domain)
Activity 1:
Question Group 1
Question 1
The AXE notation and molecular shape for three molecules is shown. Assuming all A-X bonds are polar, identify which molecule would be polar. Select all that apply.

Question 2
The AXE notation and molecular shape for three molecules is shown. Assuming all A-X bonds are polar, identify which molecule would be polar. Select all that apply.

Question 3
The AXE notation and molecular shape for three molecules is shown. Assuming all A-X bonds are polar, identify which molecule would be polar. Select all that apply.

Question Group 2
Question 4
The AXE notation and molecular shape for three molecules is shown. Assuming all A-X bonds are polar, identify which molecule would be polar. Select all that apply.

Question 5
The AXE notation and molecular shape for three molecules is shown. Assuming all A-X bonds are polar, identify which molecule would be polar. Select all that apply.

Question 6
The AXE notation and molecular shape for three molecules is shown. Assuming all A-X bonds are polar, identify which molecule would be polar. Select all that apply.

Question Group 3
Question 7
The AXE notation and molecular shape for three molecules is shown. Assuming all A-X bonds are polar, identify which molecule would be polar. Select all that apply.

Question 8
The AXE notation and molecular shape for three molecules is shown. Assuming all A-X bonds are polar, identify which molecule would be polar. Select all that apply.

Question 9
The AXE notation and molecular shape for three molecules is shown. Assuming all A-X bonds are polar, identify which molecule would be polar. Select all that apply.

Question Group 4
Question 10
The AXE notation and molecular shape for three molecules is shown. Assuming all A-X bonds are polar, identify which molecule would be polar. Select all that apply.

Question 11
The AXE notation and molecular shape for three molecules is shown. Assuming all A-X bonds are polar, identify which molecule would be polar. Select all that apply.

Question 12
The AXE notation and molecular shape for three molecules is shown. Assuming all A-X bonds are polar, identify which molecule would be polar. Select all that apply.

Question Group 5
Question 13
The AXE notation and molecular shape for three molecules is shown. Assuming all A-X bonds are polar, identify which molecule would be polar. Select all that apply.

Question 14
The AXE notation and molecular shape for three molecules is shown. Assuming all A-X bonds are polar, identify which molecule would be polar. Select all that apply.

Question 15
The AXE notation and molecular shape for three molecules is shown. Assuming all A-X bonds are polar, identify which molecule would be polar. Select all that apply.
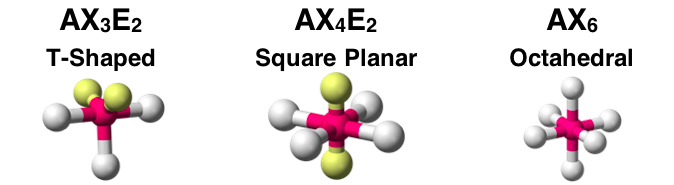
Question Group 6
Question 16
The AXE notation and molecular shape for three molecules is shown. Assuming all A-X bonds are polar, identify which molecule would be polar. Select all that apply.
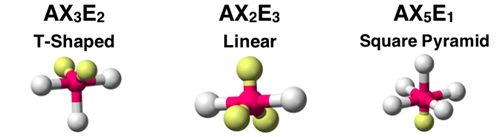
Question 17
The AXE notation and molecular shape for three molecules is shown. Assuming all A-X bonds are polar, identify which molecule would be polar. Select all that apply.
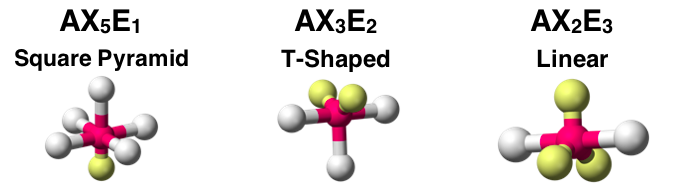
Question 18
The AXE notation and molecular shape for three molecules is shown. Assuming all A-X bonds are polar, identify which molecule would be polar. Select all that apply.

Activity 2:
 Question Group 7
Question Group 7
Question 19
The Lewis electron dot structure for NO3- is shown. Identify the molecular shape. Then indicate if the molecule is polar or non-polar.
 Question 20
Question 20
The Lewis electron dot structure for SO3 is shown. Identify the molecular shape. Then indicate if the molecule is polar or non-polar.
 Question 21
Question 21
The Lewis electron dot structure for BCl3 is shown. Identify the molecular shape. Then indicate if the molecule is polar or non-polar.
Question Group 8
 Question 22
Question 22
The Lewis electron dot structure for NO2- is shown. Identify the molecular shape. Then indicate if the molecule is polar or non-polar.
 Question 23
Question 23
The Lewis electron dot structure for SO2 is shown. Identify the molecular shape. Then indicate if the molecule is polar or non-polar.
 Question 24
Question 24
The Lewis electron dot structure for SeO2 is shown. Identify the molecular shape. Then indicate if the molecule is polar or non-polar.
Question Group 9
 Question 25
Question 25
The Lewis electron dot structure for H2O is shown. Identify the molecular shape. Then indicate if the molecule is polar or non-polar.
 Question 26
Question 26
The Lewis electron dot structure for SCl2 is shown. Identify the molecular shape. Then indicate if the molecule is polar or non-polar.
 Question 27
Question 27
The Lewis electron dot structure for OCl2 is shown. Identify the molecular shape. Then indicate if the molecule is polar or non-polar.
Question Group 10
 Question 28
Question 28
The Lewis electron dot structure for SO32- is shown. Identify the molecular shape. Then indicate if the molecule is polar or non-polar.
 Question 29
Question 29
The Lewis electron dot structure for H3O+ is shown. Identify the molecular shape. Then indicate if the molecule is polar or non-polar.
 Question 30
Question 30
The Lewis electron dot structure for PCl3 is shown. Identify the molecular shape. Then indicate if the molecule is polar or non-polar.
Question Group 11
 Question 31
Question 31
The Lewis electron dot structure for CH4 is shown. Identify the molecular shape. Then indicate if the molecule is polar or non-polar.
 Question 32
Question 32
The Lewis electron dot structure for CCl4 is shown. Identify the molecular shape. Then indicate if the molecule is polar or non-polar.
 Question 33
Question 33
The Lewis electron dot structure for CBr4 is shown. Identify the molecular shape. Then indicate if the molecule is polar or non-polar.
Question Group 12
 Question 34
Question 34
The Lewis electron dot structure for CO2 is shown. Identify the molecular shape. Then indicate if the molecule is polar or non-polar.
 Question 35
Question 35
The Lewis electron dot structure for CS2 is shown. Identify the molecular shape. Then indicate if the molecule is polar or non-polar.
 Question 36
Question 36
The Lewis electron dot structure for SiO2 is shown. Identify the molecular shape. Then indicate if the molecule is polar or non-polar.
Activity 3
 Question Group 13
Question Group 13
Question 37
The Lewis electron dot structure for XeF2 is shown. Identify the molecular shape. Then indicate if the molecule is polar or non-polar.
 Question 38
Question 38
The Lewis electron dot structure for ICl2- is shown. Identify the molecular shape. Then indicate if the molecule is polar or non-polar.
 Question 39
Question 39
The Lewis electron dot structure for IBr2- is shown. Identify the molecular shape. Then indicate if the molecule is polar or non-polar.
Question Group 14
 Question 40
Question 40
The Lewis electron dot structure for ICl3 is shown. Identify the molecular shape. Then indicate if the molecule is polar or non-polar.
 Question 41
Question 41
The Lewis electron dot structure for BrF3 is shown. Identify the molecular shape. Then indicate if the molecule is polar or non-polar.
 Question 42
Question 42
The Lewis electron dot structure for ClF3 is shown. Identify the molecular shape. Then indicate if the molecule is polar or non-polar.
Question Group 15
 Question 43
Question 43
The Lewis electron dot structure for SF4 is shown. Identify the molecular shape. Then indicate if the molecule is polar or non-polar.
 Question 44
Question 44
The Lewis electron dot structure for TeF4 is shown. Identify the molecular shape. Then indicate if the molecule is polar or non-polar.
 Question 45
Question 45
The Lewis electron dot structure for SbCl4- is shown. Identify the molecular shape. Then indicate if the molecule is polar or non-polar.
Question Group 16
 Question 46
Question 46
The Lewis electron dot structure for PCl5 is shown. Identify the molecular shape. Then indicate if the molecule is polar or non-polar.
 Question 47
Question 47
The Lewis electron dot structure for PBr5 is shown. Identify the molecular shape. Then indicate if the molecule is polar or non-polar.
 Question 48
Question 48
The Lewis electron dot structure for AsCl5 is shown. Identify the molecular shape. Then indicate if the molecule is polar or non-polar.
 Question Group 17
Question Group 17
Question 49
The Lewis electron dot structure for ClF5 is shown. Identify the molecular shape. Then indicate if the molecule is polar or non-polar.
 Question 50
Question 50
The Lewis electron dot structure for BrF5 is shown. Identify the molecular shape. Then indicate if the molecule is polar or non-polar.
 Question 51
Question 51
The Lewis electron dot structure for IF5 is shown. Identify the molecular shape. Then indicate if the molecule is polar or non-polar.
 Question Group 18
Question Group 18
Question 52
The Lewis electron dot structure for XeF4 is shown. Identify the molecular shape. Then indicate if the molecule is polar or non-polar.
 Question 53
Question 53
The Lewis electron dot structure for XeCl4 is shown. Identify the molecular shape. Then indicate if the molecule is polar or non-polar.
 Question 54
Question 54
The Lewis electron dot structure for ClF4- is shown. Identify the molecular shape. Then indicate if the molecule is polar or non-polar.