The Collision Model of Reaction Rates - Questions
The Collision Model of Reaction Rates Concept Builder is comprised of 53 questions. The questions are divided into 18 different Question Groups and spread across three activities. Questions in the same group are rather similar to one another. The Concept Builder is coded to select at random a question from each group until a student is successful with that group of questions.
There are three activities that can be engaged in through the Concept Builder. Those three activities are differentiated as follows:
- Reaction Rates: Question Groups 1-6 ... Identify how changes ini concentration, surface area, temperature, and catalyst use will affect the rate of a reaction.
- The Collision Model: Question Groups 7-12 ... Use a particle view to explain the underlying reasons why concentration changes, surface area changes, temperature changes, and the use of a catalyst will lead to changes in the reaction rate.
- Energy and Orientation: Question Groups 13-18 ... Relate the orientation of colliding particles and the activation energy (and related energy-factors) to the likelihood of there being an effective collision.
The questions from each group are shown below. Teachers are encouraged to view the questions in order to judge which activities are most appropriate for their classes. We recommend providing students two or more options.
The Physics Classroom grants teachers and other users the right to print these questions for private use. Users are also granted the right to copy the text and modify it for their own use. However, this document should not be uploaded to other servers for distribution to and/or display by others. The Physics Classroom website should remain the only website or server from which the document is distributed or displayed. We also provide a PDF that teachers can use under the same conditions. We have included a link to the PDF near the bottom of this page.
Collision Model of Reaction Rates
Activity 1: Reaction Rate
Question Group 1
Question 1
Zinc reacts with aqueous hydrochloric acid in a single replacement reaction to produce hydrogen gas. Three trials are conducted with varying HCl concentration. Rank the three trials in order of increasing rate of hydrogen gas production.
Question 2
Zinc reacts with aqueous hydrochloric acid in a single replacement reaction to produce hydrogen gas. Three trials are conducted with varying HCl concentration. Rank the three trials in order of increasing rate of hydrogen gas production.
Question 3
Zinc reacts with aqueous hydrochloric acid in a single replacement reaction to produce hydrogen gas. Three trials are conducted with varying HCl concentration. Rank the three trials in order of increasing rate of hydrogen gas production.
Question Group 2
Question 4
As an Alka Seltzer® dissolves in water, a reaction occurs to produce carbon dioxide gas. Three trials are conducted with varying water temperatures. Rank the three trials in order of increasing rate of carbon dioxide gas production.
Question 5
As an Alka Seltzer® dissolves in water, a reaction occurs to produce carbon dioxide gas. Three trials are conducted with varying water temperatures. Rank the three trials in order of increasing rate of carbon dioxide gas production.
Question 6
As an Alka Seltzer® dissolves in water, a reaction occurs to produce carbon dioxide gas. Three trials are conducted with varying water temperatures. Rank the three trials in order of increasing rate of carbon dioxide gas production.
Question Group 3
Question 7
As an Alka Seltzer® dissolves in water, a reaction occurs to produce carbon dioxide gas. Three trials are conducted with a single tablet in a beaker of 300 mL of water. The dependent variable between trials is the degree to which the tablet is ground up - whole tablet (unground), several chunks, and finely ground. Rank the three trials in order of increasing rate of carbon dioxide gas production.
Question 8
As an Alka Seltzer® dissolves in water, a reaction occurs to produce carbon dioxide gas. Three trials are conducted with a single tablet in a beaker of 300 mL of water. The dependent variable between trials is the degree to which the tablet is ground up - whole tablet (unground), several chunks, and finely ground. Rank the three trials in order of increasing rate of carbon dioxide gas production.
Question 9
As an Alka Seltzer® dissolves in water, a reaction occurs to produce carbon dioxide gas. Three trials are conducted with a single tablet in a beaker of 300 mL of water. The dependent variable between trials is the degree to which the tablet is ground up - whole tablet (unground), several chunks, and finely ground. Rank the three trials in order of increasing rate of carbon dioxide gas production.
Question Group 4
 Question 10
Question 10
Line 1 on the graph at the right represents the changes in product concentration as a reaction takes place over the course of time. If a
catalystis added to the reactants, then one would expect the graph to look like ...
 Question 11
Question 11
Line 1 on the graph at the right represents the changes in product concentration as a reaction takes place over the course of time. If a
catalystis added to the reactants, then one would expect the graph to look like ...
 Question 12
Question 12
Line 1 on the graph at the right represents the changes in product concentration as a reaction takes place over the course of time. If a
catalystis added to the reactants, then one would expect the graph to look like ...
Question Group 5
Question 13
Consider the four different variable changes made below. Identify any change that would not result in an increase in the reaction rate. Select all that apply.
Question 14
Consider the four different variable changes made below. Identify any change that would not result in an increase in the reaction rate. Select all that apply.
Question 15
Consider the four different variable changes made below. Identify any change that would not result in an increase in the reaction rate. Select all that apply.
Question Group 6
Question 16
Consider the four different variable changes made below. Identify any change that would not result in an increase in the reaction rate. Select all that apply.
Question 17
Consider the four different variable changes made below. Identify any change that would not result in an increase in the reaction rate. Select all that apply.
Question 18
Consider the four different variable changes made below. Identify any change that would not result in an increase in the reaction rate. Select all that apply.
Activity 2: Collision Theory
Question Group 7
Question 19
Grinding solid reactants into a fine power will increase reaction rates. Which statement best describes the reason for this increase in reaction rate?
Grinding increases surface area and exposes a greater portion of the reactants to potential collisions.
Grinding reactants makes reactant particles more energetic resulting in more particles meeting the energy requirement.
Grinding increases the activation energy for the reaction, resulting in more particles meeting the energy requirement.
Grinding the solid results in a reaction pathway that has a lowered activation energy.
Question 20
Grinding solid reactants into a fine power will increase reaction rates. Which statement best describes the reason for this increase in reaction rate?
Grinding the solid results in a reaction pathway that has a lowered activation energy.
Grinding reactants makes reactant particles more energetic resulting in more particles meeting the energy requirement.
Grinding increases surface area and exposes a greater portion of the reactants to potential collisions.
Grinding increases the activation energy for the reaction, resulting in more particles meeting the energy requirement.
Question 21
Grinding solid reactants into a fine power will increase reaction rates. Which statement best describes the reason for this increase in reaction rate?
Grinding increases the activation energy for the reaction, resulting in more particles meeting the energy requirement.
Grinding reactants makes reactant particles more energetic resulting in more particles meeting the energy requirement.
Grinding the solid results in a reaction pathway that has a lowered activation energy.
Grinding increases surface area and exposes a greater portion of the reactants to potential collisions.
Question Group 8
Question 22
Using a catalyst causes the reaction rate to increase. Which statement best explains why the use of a catalyst increases reaction rate?
Catalysts cause increases in reactant concentrations and a higher frequency of collisions.
Using a catalyst alters the reaction pathway, resulting in one with a lower activation energy.
Catalysts increase the temperature of a reaction vessel, resulting in more energetic reactant particles.
Catalysts increase the energy of activation, thus causing a higher frequency of collisions.
Question 23
Using a catalyst causes the reaction rate to increase. Which statement best explains why the use of a catalyst increases reaction rate?
Catalysts increase the energy of activation, thus causing a higher frequency of collisions.
Catalysts cause increases in reactant concentrations and a higher frequency of collisions.
Using a catalyst alters the reaction pathway, resulting in one with a lower activation energy.
Catalysts increase the temperature of a reaction vessel, resulting in more energetic reactant particles.
Question 24
Using a catalyst causes the reaction rate to increase. Which statement best explains why the use of a catalyst increases reaction rate?
Catalysts cause increases in reactant concentrations and a higher frequency of collisions.
Catalysts increase the energy of activation, thus causing a higher frequency of collisions.
Catalysts increase the temperature of a reaction vessel, resulting in more energetic reactant particles.
Using a catalyst alters the reaction pathway, resulting in one with a lower activation energy.
Question Group 9
Question 25
Reactions occur more rapidly at higher temperatures. Which
twostatements best explain why increasing temperatures increase reaction rates? Pick two statements.
A greater number of particles meet the energy requirement for an effective collision.
Particle speeds increase, resulting in a higher collision frequency.
Solid reactants assume larger grain size (less powdery) with greater surface area.
Higher temperatures cause reactant particles to become more concentrated.
The activation energy is lowered by increasing temperatures.
Question 26
Reactions occur more rapidly at higher temperatures. Which
twostatements best explain why increasing temperatures increase reaction rates? Pick two statements.
Higher temperatures cause reactant particles to become more concentrated.
Particle speeds increase, resulting in a higher collision frequency.
The activation energy is lowered by increasing temperatures.
Solid reactants assume larger grain size (less powdery) with greater surface area.
A greater number of particles meet the energy requirement for an effective collision.
Question 27
Reactions occur more rapidly at higher temperatures. Which
twostatements best explain why increasing temperatures increase reaction rates? Pick two statements.
Particle speeds increase, resulting in a higher collision frequency.
Solid reactants assume larger grain size (less powdery) with greater surface area.
A greater number of particles meet the energy requirement for an effective collision.
Higher temperatures cause reactant particles to become more concentrated.
The activation energy is lowered by increasing temperatures.
Question Group 10
Question 28
Increasing reactant concentrations cause reaction rates to increase. Which statement best explains why increases in concentrations cause reactions to run faster?
At higher concentrations, reactant particles collide more frequently.
Particles
feed off of each other's energyand become more energetic at high concentrations.
Surface area increases as reactant concentration increases, thus increasing the collision frequency.
High concentrations cause decreases in the average activation energy.
Question 29
Increasing reactant concentrations cause reaction rates to increase. Which statement best explains why increases in concentrations cause reactions to run faster?
High concentrations cause decreases in the average activation energy.
Surface area increases as reactant concentration increases, thus increasing the collision frequency.
Particles
feed off of each other's energyand become more energetic at high concentrations.
At higher concentrations, reactant particles collide more frequently.
Question 30
Increasing reactant concentrations cause reaction rates to increase. Which statement best explains why increases in concentrations cause reactions to run faster?
Surface area increases as reactant concentration increases, thus increasing the collision frequency.
At higher concentrations, reactant particles collide more frequently.
High concentrations cause decreases in the average activation energy.
Particles
feed off of each other's energyand become more energetic at high concentrations.
Question Group 11
Question 31
Three characteristics of reactants or reactions are described below. Which one would
not result in an increase in the rate of reaction?
Question 32
Three characteristics of reactants or reactions are described below. Which one would
not result in an increase in the rate of reaction?
Question 33
Three characteristics of reactants or reactions are described below. Which one would
not result in an increase in the rate of reaction?
Question Group 12
Question 34
Three characteristics of reactants or reactions are described below. Which one would result in an increase in the rate of reaction?
Question 35
Three characteristics of reactants or reactions are described below. Which one would result in an increase in the rate of reaction?
Question 36
Three characteristics of reactants or reactions are described below. Which one would result in an increase in the rate of reaction?
Activity 3: Orientation and Energy
Question Group 13
Question 37
According to the Collision Model of Reaction Rates, reactant particles must collide in order for bonds to break and products to form. But not every collision results in a reaction. The model proposes that a collision will effecively form products if it meets two requirements. Which statements describe those requirements? Pick two
The colliding particles must have the proper geometric orientation.
The energy of colliding particles must exceed the activation energy.
The reactants must have a lower bond energy than that of the products.
The reaction must be exothermic and release energy to the surroundings.
The number of bonds in reactant particles must be less than that of products.
Question 38
According to the Collision Model of Reaction Rates, reactant particles must collide in order for bonds to break and products to form. But not every collision results in a reaction. The model proposes that a collision will effecively form products if it meets two requirements. Which statements describe those requirements? Pick two
The reaction must be exothermic and release energy to the surroundings.
The number of bonds in reactant particles must be less than that of products.
The energy of colliding particles must exceed the activation energy.
The reactants must have a lower bond energy than that of the products.
The colliding particles must have the proper geometric orientation.
Question 39
According to the Collision Model of Reaction Rates, reactant particles must collide in order for bonds to break and products to form. But not every collision results in a reaction. The model proposes that a collision will effecively form products if it meets two requirements. Which statements describe those requirements? Pick two
The number of bonds in reactant particles must be less than that of products.
The colliding particles must have the proper geometric orientation.
The reactants must have a lower bond energy than that of the products.
The reaction must be exothermic and release energy to the surroundings.
The energy of colliding particles must exceed the activation energy.
Question Group 14
Question 40
A particle representation of a reaction step is shown.
Which one of the collision orientations would most likely result in the breaking of a N-O bond and the formation of a new O-O bond?
Question 41
A particle representation of a reaction step is shown.
Which one of the collision orientations would most likely result in the breaking of a N-O bond and the formation of a new O-O bond?
Question 42
A particle representation of a reaction step is shown.
Which one of the collision orientations would most likely result in the breaking of a N-O bond and the formation of a new O-O bond?
Question Group 15
Question 43
Carbon monoxide reacts with nitrogen dioxide to produce carbon dioxide and nitrogen monoxide. A particle representation of the reaction is shown.
Which one of the collision orientations would most likely result in the breaking of a N-O bond and the formation of a new C-O bond?
Question 44
Carbon monoxide reacts with nitrogen dioxide to produce carbon dioxide and nitrogen monoxide. A particle representation of the reaction is shown.
Which one of the collision orientations would most likely result in the breaking of a N-O bond and the formation of a new C-O bond?
Question 45
Carbon monoxide reacts with nitrogen dioxide to produce carbon dioxide and nitrogen monoxide. A particle representation of the reaction is shown.
Which one of the collision orientations would most likely result in the breaking of a N-O bond and the formation of a new C-O bond?
Question Group 16
 Question 46
Question 46
The energy profile for reactants turning into products is shown. The activation energy (E
a) for this reaction is _____________ kJ/mol.
100
200
300
400
500
 Question 47
Question 47
The energy profile for reactants turning into products is shown. The activation energy (E
a) for this reaction is _____________ kJ/mol.
100
200
400
500
600
 Question 48
Question 48
The energy profile for reactants turning into products is shown. The activation energy (E
a) for this reaction is _____________ kJ/mol.
200
300
500
700
800
Question Group 18
 Question 49
Question 49
A reaction can take place by two different mechanisms or pathways - referred to as
Pathway A and
Pathway B. The energy profile for each pathway is shown. When comparing
Pathway A to
Pathway B, one would conclude that ...
A. ... the enthalphy change is ....
the same for each pathway
greater for Pathway A
greater for Pathway B
B. ... the activation energy is ...
the same for each pathway
greater for Pathway A
greater for Pathway B
C. ... the catalyzed pathway is ...
Pathway A
Pathway B.
 Question 50
Question 50
A reaction can take place by two different mechanisms or pathways - referred to as
Pathway Aand
Pathway B. The energy profile for each pathway is shown. When comparing
Pathway Ato
Pathway B, one would conclude that ...
A. ... the enthalphy change is ....
the same for each pathway
greater for Pathway A
greater for Pathway B
B. ... the activation energy is ...
the same for each pathway
greater for Pathway A
greater for Pathway B
C. ... the catalyzed pathway is ...
Pathway A
Pathway B.
 Question 51
Question 51
A reaction can take place by two different mechanisms or pathways - referred to as
Pathway Aand
Pathway B. The energy profile for each pathway is shown. When comparing
Pathway Ato
Pathway B, one would conclude that ...
A. ... the enthalphy change is ....
the same for each pathway
greater for Pathway A
greater for Pathway B
B. ... the activation energy is ...
the same for each pathway
greater for Pathway A
greater for Pathway B
C. ... the catalyzed pathway is ...
Pathway A
Pathway B.
Question Group 18
 Question 52
Question 52
Not every particle in a sample has the same kinetic energy (KE). There is a distribution of KE values within the sample. The graphs are KE distribution curves for two samples -
Sample Aand
Sample B- of the
same reactantsat
two different temperatures. The activation energy (
Ea) is marked on the graph. When comparing the curves for the two samples, one can conclude that ...
A. ... the number of particles whose KE exceeds the activation energy is
the same in each sample
greater in Sample A
greater in Sample B
B. ... the temperature is greatest in
sample A
sample B
C. ... the reaction will occur at a greater rate in
sample A
sample B
Question 53
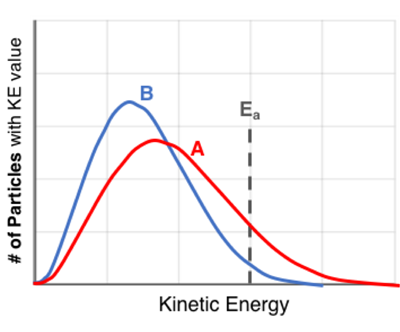
Not every particle in a sample has the same kinetic energy (KE). There is a distribution of KE values within the sample. The graphs are KE distribution curves for two samples -
Sample Aand
Sample B- of the
same reactantsat
two different temperatures. The activation energy (
Ea) is marked on the graph. When comparing the curves for the two samples, one can conclude that ...
A. ... the number of particles whose KE exceeds the activation energy is
the same in each sample
greater in Sample A
greater in Sample B
B. ... the temperature is greatest in
sample A
sample B
C. ... the reaction will occur at a greater rate in
sample A
sample B