Intermolecular Forces - Questions
The Intermolecular Forces Concept Builder is comprised of 39 questions. The questions are divided into 13 different Question Groups and spread across three different activities. Questions in the same group are rather similar to one another. The Concept Builder is coded to select at random a question from each group until a student is successful with that group of questions.
There are three activities in the Concept Builder. Those three activities are differentiated as follows:
- Paragraph Completion: Question Groups 1-3 ... Complete a paragraph by selecting words and phrases from a bank. Each paragraph targets a different intermolecular force type - London dispersion forces, dipole-dipole interactions, and hydrogen bonding.
- Force Identification: Question Groups 4-9 ... Given a Lewis electron dot structure for a substance, identify if there are polar bonds present, determine if the molecule is polar or non-polar, and identify the types of intermolecular forces in the substance.
- Match That Color: Question Groups 10-13 ... Given the formula of three strategically-chosen substances, rank the substances in order of the strength of their intermolecular forces.
The questions from each group are shown below. Teachers are encouraged to view the questions in order to judge which difficulty levels are most appropriate for their classes. We recommend providing students two or more options.
The Physics Classroom grants teachers and other users the right to print these questions for private use. Users are also granted the right to copy the text and modify it for their own use. However, this document should not be uploaded to other servers for distribution to and/or display by others. The Physics Classroom website should remain the only website or server from which the document is distributed or displayed. We also provide a PDF that teachers can use under the same conditions. We have included a link to the PDF near the bottom of this page.
Intermolecular Forces
Activity 1: Paragraph Completion
Question Group 1
Question 1
Fill in the blanks to complete the following paragraph about dipole-dipole interactions. Tap a blank to toggle through answer options.
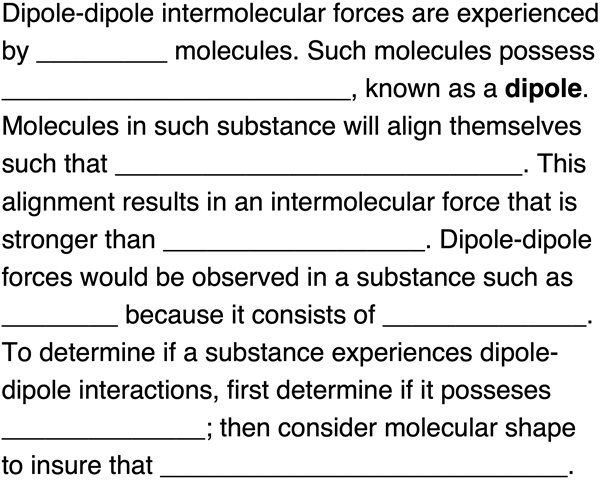
Question Group 2
Question 2
Fill in the blanks to complete the following paragraph about London dispersion forces. Tap a blank to toggle through answer options.
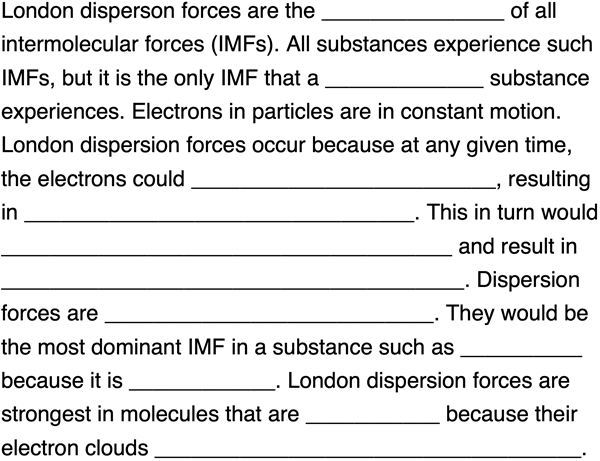
Question Group 3
Question 3
Fill in the blanks to complete the following paragraph about hydrogen bonding. Tap a blank to toggle through answer options.
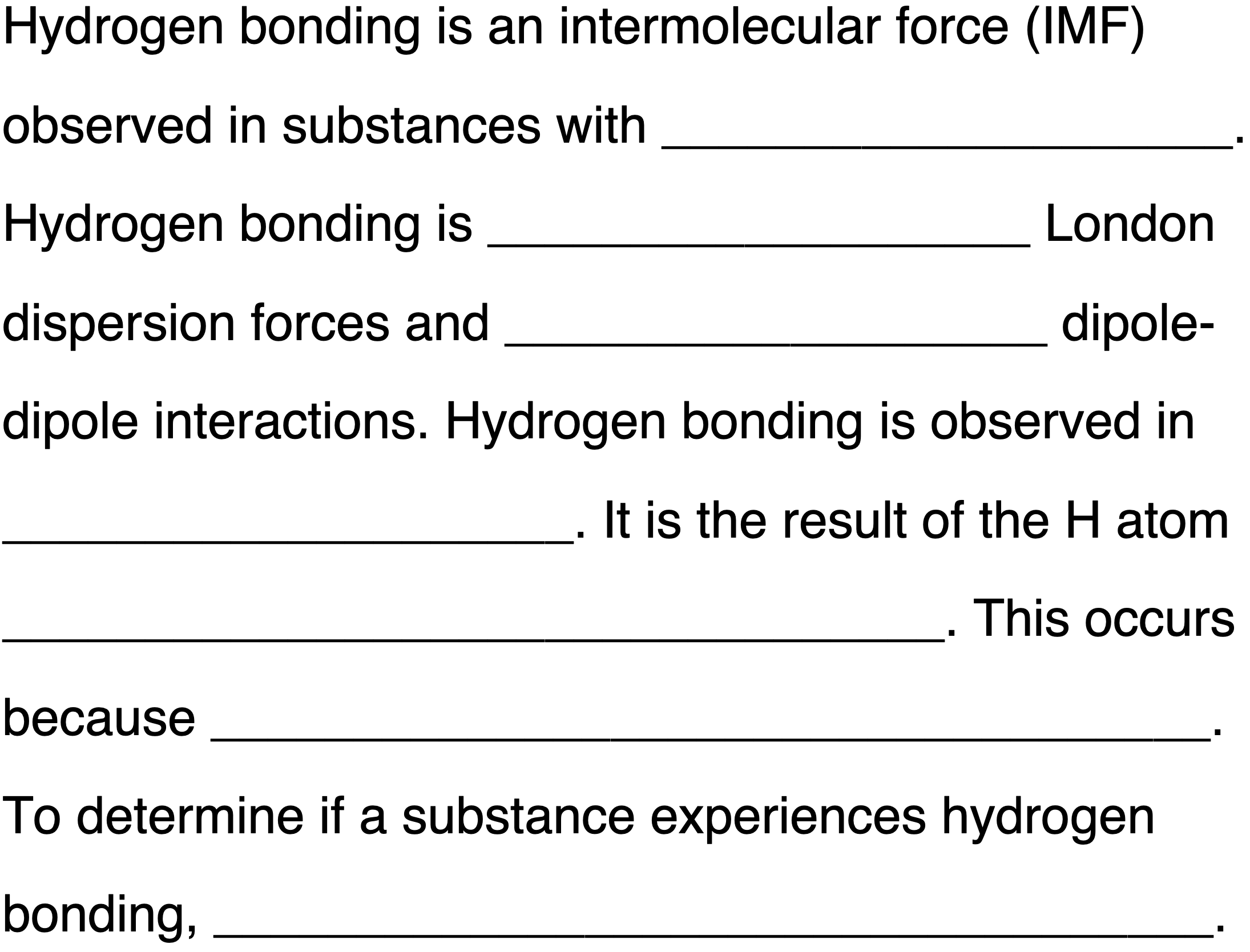
Activity 2: Force Identification
Question Group 4
Question 4
 A formula and Lewis Structure for a substance is shown. Identify whether it has polar bonds. Identify whether the molecule is a polar molecule. And identify all the types of intermolecular forces (IMFs) that the substance would experience.
A formula and Lewis Structure for a substance is shown. Identify whether it has polar bonds. Identify whether the molecule is a polar molecule. And identify all the types of intermolecular forces (IMFs) that the substance would experience.
Polar Bonds? Yes No
Polar Molecule? Yes No
Types of Intermolecular Forces (circle all that apply):
London dispersion forces
Dipole-dipole interactions
Hydrogen bonding
Question 5
 A formula and Lewis Structure for a substance is shown. Identify whether it has polar bonds. Identify whether the molecule is a polar molecule. And identify all the types of intermolecular forces (IMFs) that the substance would experience.
A formula and Lewis Structure for a substance is shown. Identify whether it has polar bonds. Identify whether the molecule is a polar molecule. And identify all the types of intermolecular forces (IMFs) that the substance would experience.
Polar Bonds? Yes No
Polar Molecule? Yes No
Types of Intermolecular Forces (circle all that apply):
London dispersion forces
Dipole-dipole interactions
Hydrogen bonding
Question 6
 A formula and Lewis Structure for a substance is shown. Identify whether it has polar bonds. Identify whether the molecule is a polar molecule. And identify all the types of intermolecular forces (IMFs) that the substance would experience.
A formula and Lewis Structure for a substance is shown. Identify whether it has polar bonds. Identify whether the molecule is a polar molecule. And identify all the types of intermolecular forces (IMFs) that the substance would experience.
Polar Bonds? Yes No
Polar Molecule? Yes No
Types of Intermolecular Forces (circle all that apply):
London dispersion forces
Dipole-dipole interactions
Hydrogen bonding
Question 7
 A formula and Lewis Structure for a substance is shown. Identify whether it has polar bonds. Identify whether the molecule is a polar molecule. And identify all the types of intermolecular forces (IMFs) that the substance would experience.
A formula and Lewis Structure for a substance is shown. Identify whether it has polar bonds. Identify whether the molecule is a polar molecule. And identify all the types of intermolecular forces (IMFs) that the substance would experience.
Polar Bonds? Yes No
Polar Molecule? Yes No
Types of Intermolecular Forces (circle all that apply):
London dispersion forces
Dipole-dipole interactions
Hydrogen bonding
Question Group 5 was removed from the Concept Builder on 12/5/2024
Question Group 5
Question 8
 A formula and Lewis Structure for a substance is shown. Identify whether it has polar bonds. Identify whether the molecule is a polar molecule. And identify all the types of intermolecular forces (IMFs) that the substance would experience.
A formula and Lewis Structure for a substance is shown. Identify whether it has polar bonds. Identify whether the molecule is a polar molecule. And identify all the types of intermolecular forces (IMFs) that the substance would experience.
Polar Bonds? Yes No
Polar Molecule? Yes No
Types of Intermolecular Forces (circle all that apply):
London dispersion forces
Dipole-dipole interactions
Hydrogen bonding
Question 9
 A formula and Lewis Structure for a substance is shown. Identify whether it has polar bonds. Identify whether the molecule is a polar molecule. And identify all the types of intermolecular forces (IMFs) that the substance would experience.
A formula and Lewis Structure for a substance is shown. Identify whether it has polar bonds. Identify whether the molecule is a polar molecule. And identify all the types of intermolecular forces (IMFs) that the substance would experience.
Polar Bonds? Yes No
Polar Molecule? Yes No
Types of Intermolecular Forces (circle all that apply):
London dispersion forces
Dipole-dipole interactions
Hydrogen bonding
Question 10
 A formula and Lewis Structure for a substance is shown. Identify whether it has polar bonds. Identify whether the molecule is a polar molecule. And identify all the types of intermolecular forces (IMFs) that the substance would experience.
A formula and Lewis Structure for a substance is shown. Identify whether it has polar bonds. Identify whether the molecule is a polar molecule. And identify all the types of intermolecular forces (IMFs) that the substance would experience.
Polar Bonds? Yes No
Polar Molecule? Yes No
Types of Intermolecular Forces (circle all that apply):
London dispersion forces
Dipole-dipole interactions
Hydrogen bonding
Question 11
 A formula and Lewis Structure for a substance is shown. Identify whether it has polar bonds. Identify whether the molecule is a polar molecule. And identify all the types of intermolecular forces (IMFs) that the substance would experience.
A formula and Lewis Structure for a substance is shown. Identify whether it has polar bonds. Identify whether the molecule is a polar molecule. And identify all the types of intermolecular forces (IMFs) that the substance would experience.
Polar Bonds? Yes No
Polar Molecule? Yes No
Types of Intermolecular Forces (circle all that apply):
London dispersion forces
Dipole-dipole interactions
Hydrogen bonding
Question Group 6
Question 12
 A formula and Lewis Structure for a substance is shown. Identify whether it has polar bonds. Identify whether the molecule is a polar molecule. And identify all the types of intermolecular forces (IMFs) that the substance would experience.
A formula and Lewis Structure for a substance is shown. Identify whether it has polar bonds. Identify whether the molecule is a polar molecule. And identify all the types of intermolecular forces (IMFs) that the substance would experience.
Polar Bonds? Yes No
Polar Molecule? Yes No
Types of Intermolecular Forces (circle all that apply):
London dispersion forces
Dipole-dipole interactions
Hydrogen bonding
Question 13
 A formula and Lewis Structure for a substance is shown. Identify whether it has polar bonds. Identify whether the molecule is a polar molecule. And identify all the types of intermolecular forces (IMFs) that the substance would experience.
A formula and Lewis Structure for a substance is shown. Identify whether it has polar bonds. Identify whether the molecule is a polar molecule. And identify all the types of intermolecular forces (IMFs) that the substance would experience.
Polar Bonds? Yes No
Polar Molecule? Yes No
Types of Intermolecular Forces (circle all that apply):
London dispersion forces
Dipole-dipole interactions
Hydrogen bonding
Question 14
 A formula and Lewis Structure for a substance is shown. Identify whether it has polar bonds. Identify whether the molecule is a polar molecule. And identify all the types of intermolecular forces (IMFs) that the substance would experience.
A formula and Lewis Structure for a substance is shown. Identify whether it has polar bonds. Identify whether the molecule is a polar molecule. And identify all the types of intermolecular forces (IMFs) that the substance would experience.
Polar Bonds? Yes No
Polar Molecule? Yes No
Types of Intermolecular Forces (circle all that apply):
London dispersion forces
Dipole-dipole interactions
Hydrogen bonding
Question 15
 A formula and Lewis Structure for a substance is shown. Identify whether it has polar bonds. Identify whether the molecule is a polar molecule. And identify all the types of intermolecular forces (IMFs) that the substance would experience.
A formula and Lewis Structure for a substance is shown. Identify whether it has polar bonds. Identify whether the molecule is a polar molecule. And identify all the types of intermolecular forces (IMFs) that the substance would experience.
Polar Bonds? Yes No
Polar Molecule? Yes No
Types of Intermolecular Forces (circle all that apply):
London dispersion forces
Dipole-dipole interactions
Hydrogen bonding
Question Group 7
Question 16
 A formula and Lewis Structure for a substance is shown. Identify whether it has polar bonds. Identify whether the molecule is a polar molecule. And identify all the types of intermolecular forces (IMFs) that the substance would experience.
A formula and Lewis Structure for a substance is shown. Identify whether it has polar bonds. Identify whether the molecule is a polar molecule. And identify all the types of intermolecular forces (IMFs) that the substance would experience.
Polar Bonds? Yes No
Polar Molecule? Yes No
Types of Intermolecular Forces (circle all that apply):
London dispersion forces
Dipole-dipole interactions
Hydrogen bonding
Question 17
 A formula and Lewis Structure for a substance is shown. Identify whether it has polar bonds. Identify whether the molecule is a polar molecule. And identify all the types of intermolecular forces (IMFs) that the substance would experience.
A formula and Lewis Structure for a substance is shown. Identify whether it has polar bonds. Identify whether the molecule is a polar molecule. And identify all the types of intermolecular forces (IMFs) that the substance would experience.
Polar Bonds? Yes No
Polar Molecule? Yes No
Types of Intermolecular Forces (circle all that apply):
London dispersion forces
Dipole-dipole interactions
Hydrogen bonding
Question 18
 A formula and Lewis Structure for a substance is shown. Identify whether it has polar bonds. Identify whether the molecule is a polar molecule. And identify all the types of intermolecular forces (IMFs) that the substance would experience.
A formula and Lewis Structure for a substance is shown. Identify whether it has polar bonds. Identify whether the molecule is a polar molecule. And identify all the types of intermolecular forces (IMFs) that the substance would experience.
Polar Bonds? Yes No
Polar Molecule? Yes No
Types of Intermolecular Forces (circle all that apply):
London dispersion forces
Dipole-dipole interactions
Hydrogen bonding
Question 19
 A formula and Lewis Structure for a substance is shown. Identify whether it has polar bonds. Identify whether the molecule is a polar molecule. And identify all the types of intermolecular forces (IMFs) that the substance would experience.
A formula and Lewis Structure for a substance is shown. Identify whether it has polar bonds. Identify whether the molecule is a polar molecule. And identify all the types of intermolecular forces (IMFs) that the substance would experience.
Polar Bonds? Yes No
Polar Molecule? Yes No
Types of Intermolecular Forces (circle all that apply):
London dispersion forces
Dipole-dipole interactions
Hydrogen bonding
Question Group 8
Question 20
 A formula and Lewis Structure for a substance is shown. Identify whether it has polar bonds. Identify whether the molecule is a polar molecule. And identify all the types of intermolecular forces (IMFs) that the substance would experience.
A formula and Lewis Structure for a substance is shown. Identify whether it has polar bonds. Identify whether the molecule is a polar molecule. And identify all the types of intermolecular forces (IMFs) that the substance would experience.
Polar Bonds? Yes No
Polar Molecule? Yes No
Types of Intermolecular Forces (circle all that apply):
London dispersion forces
Dipole-dipole interactions
Hydrogen bonding
Question 21
 A formula and Lewis Structure for a substance is shown. Identify whether it has polar bonds. Identify whether the molecule is a polar molecule. And identify all the types of intermolecular forces (IMFs) that the substance would experience.
A formula and Lewis Structure for a substance is shown. Identify whether it has polar bonds. Identify whether the molecule is a polar molecule. And identify all the types of intermolecular forces (IMFs) that the substance would experience.
Polar Bonds? Yes No
Polar Molecule? Yes No
Types of Intermolecular Forces (circle all that apply):
London dispersion forces
Dipole-dipole interactions
Hydrogen bonding
Question 22
 A formula and Lewis Structure for a substance is shown. Identify whether it has polar bonds. Identify whether the molecule is a polar molecule. And identify all the types of intermolecular forces (IMFs) that the substance would experience.
A formula and Lewis Structure for a substance is shown. Identify whether it has polar bonds. Identify whether the molecule is a polar molecule. And identify all the types of intermolecular forces (IMFs) that the substance would experience.
Polar Bonds? Yes No
Polar Molecule? Yes No
Types of Intermolecular Forces (circle all that apply):
London dispersion forces
Dipole-dipole interactions
Hydrogen bonding
Question 23
 A formula and Lewis Structure for a substance is shown. Identify whether it has polar bonds. Identify whether the molecule is a polar molecule. And identify all the types of intermolecular forces (IMFs) that the substance would experience.
A formula and Lewis Structure for a substance is shown. Identify whether it has polar bonds. Identify whether the molecule is a polar molecule. And identify all the types of intermolecular forces (IMFs) that the substance would experience.
Polar Bonds? Yes No
Polar Molecule? Yes No
Types of Intermolecular Forces (circle all that apply):
London dispersion forces
Dipole-dipole interactions
Hydrogen bonding
Question Group 9
Question 24
 A formula and Lewis Structure for a substance is shown. Identify whether it has polar bonds. Identify whether the molecule is a polar molecule. And identify all the types of intermolecular forces (IMFs) that the substance would experience.
A formula and Lewis Structure for a substance is shown. Identify whether it has polar bonds. Identify whether the molecule is a polar molecule. And identify all the types of intermolecular forces (IMFs) that the substance would experience.
Polar Bonds? Yes No
Polar Molecule? Yes No
Types of Intermolecular Forces (circle all that apply):
London dispersion forces
Dipole-dipole interactions
Hydrogen bonding
Question 25
 A formula and Lewis Structure for a substance is shown. Identify whether it has polar bonds. Identify whether the molecule is a polar molecule. And identify all the types of intermolecular forces (IMFs) that the substance would experience.
A formula and Lewis Structure for a substance is shown. Identify whether it has polar bonds. Identify whether the molecule is a polar molecule. And identify all the types of intermolecular forces (IMFs) that the substance would experience.
Polar Bonds? Yes No
Polar Molecule? Yes No
Types of Intermolecular Forces (circle all that apply):
London dispersion forces
Dipole-dipole interactions
Hydrogen bonding
Question 26
 A formula and Lewis Structure for a substance is shown. Identify whether it has polar bonds. Identify whether the molecule is a polar molecule. And identify all the types of intermolecular forces (IMFs) that the substance would experience.
A formula and Lewis Structure for a substance is shown. Identify whether it has polar bonds. Identify whether the molecule is a polar molecule. And identify all the types of intermolecular forces (IMFs) that the substance would experience.
Polar Bonds? Yes No
Polar Molecule? Yes No
Types of Intermolecular Forces (circle all that apply):
London dispersion forces
Dipole-dipole interactions
Hydrogen bonding
Question 27
 A formula and Lewis Structure for a substance is shown. Identify whether it has polar bonds. Identify whether the molecule is a polar molecule. And identify all the types of intermolecular forces (IMFs) that the substance would experience.
A formula and Lewis Structure for a substance is shown. Identify whether it has polar bonds. Identify whether the molecule is a polar molecule. And identify all the types of intermolecular forces (IMFs) that the substance would experience.
Polar Bonds? Yes No
Polar Molecule? Yes No
Types of Intermolecular Forces (circle all that apply):
London dispersion forces
Dipole-dipole interactions
Hydrogen bonding
Activity 3: Ranking Tasks
Question Group 10
Question 28
For the following substances, consider the types of intermolecular forces and their relative strength. Then rank the substances in terms of the overall strength of the intermolecular forces.
NH3
PH3
AsH3
Question 29
For the following substances, consider the types of intermolecular forces and their relative strength. Then rank the substances in terms of the overall strength of the intermolecular forces.
AsH3
NH3
PH3
Question 30
For the following substances, consider the types of intermolecular forces and their relative strength. Then rank the substances in terms of the overall strength of the intermolecular forces.
PH3
AsH3
NH3
Question Group 11
Question 31
For the following substances, consider the types of intermolecular forces and their relative strength. Then rank the substances in terms of the overall strength of the intermolecular forces.
CH4
C4H10
C25H52
Question 32
For the following substances, consider the types of intermolecular forces and their relative strength. Then rank the substances in terms of the overall strength of the intermolecular forces.
C25H52
CH4
C4H10
Question 33
For the following substances, consider the types of intermolecular forces and their relative strength. Then rank the substances in terms of the overall strength of the intermolecular forces.
C4H10
C25H52
CH4
Question Group 12
Question 34
For the following substances, consider the types of intermolecular forces and their relative strength. Then rank the substances in terms of the overall strength of the intermolecular forces.
F2
Br2
I2
Question 35
For the following substances, consider the types of intermolecular forces and their relative strength. Then rank the substances in terms of the overall strength of the intermolecular forces.
I2
F2
Br2
Question 36
For the following substances, consider the types of intermolecular forces and their relative strength. Then rank the substances in terms of the overall strength of the intermolecular forces.
Br2
I2
F2
Question Group 13
Question 37
For the following substances, consider the types of intermolecular forces and their relative strength. Then rank the substances in terms of the overall strength of the intermolecular forces.
CH4
NH3
H2O
Question 38
For the following substances, consider the types of intermolecular forces and their relative strength. Then rank the substances in terms of the overall strength of the intermolecular forces.
H2O
CH4
NH3
Question 39
For the following substances, consider the types of intermolecular forces and their relative strength. Then rank the substances in terms of the overall strength of the intermolecular forces.
NH3
H2O
CH4