Significant Digits and Measurements - Questions
The Significant Digits and Measurement Concept Builder is comprised of 45 questions. The questions are divided into 15 different Question Groups and spread across three activities. Questions in the same group are rather similar to one another. The Concept Builder is coded to select at random a question from each group until a student is successful with that group of questions.
There are three activities in the Concept Builder. Those three activities are differentiated as follows:
- What's Significantl: Question Groups 1-6 ... Identify the proper number of significant digits in a reported value.
- Measurement: Question Groups 7-12 ... Use a measurement tool to determine a measured value to the proper number of significant digits.
- Math Operations: Question Groups 13-15 ... Use two reported measurements to calculate a numerical value to the proper number of significant digits.
The questions from each group are shown below. Teachers are encouraged to view the questions in order to judge which activities make the best fit with their curriculum.
The Physics Classroom grants teachers and other users the right to print these questions for private use. Users are also granted the right to copy the text and modify it for their own use. However, this document should not be uploaded to other servers for distribution to and/or display by others. The Physics Classroom website should remain the only website or server from which the document is distributed or displayed. We also provide a PDF that teachers can use under the same conditions. We have included a link to the PDF near the bottom of this page.
Significant Digits
Activity 1: What’s Significant?
Question Group 1
Question 1
Identify the number of significant digits in the following number:
438
Question 2
Identify the number of significant digits in the following number:
6492
Question 3
Identify the number of significant digits in the following number:
76549
Question Group 2
Question 4
Identify the number of significant digits in the following number:
380
Question 5
Identify the number of significant digits in the following number:
4520
Question 6
Identify the number of significant digits in the following number:
65930
Question Group 3
Question 7
Identify the number of significant digits in the following number:
605.2
Question 8
Identify the number of significant digits in the following number:
5806.4
Question 9
Identify the number of significant digits in the following number:
702.8
Question Group 4
Question 10
Identify the number of significant digits in the following number:
405.10
Question 11
Identify the number of significant digits in the following number:
380.20
Question 12
Identify the number of significant digits in the following number:
450.30
Question Group 5
Question 13
Identify the number of significant digits in the following number:
2030.
Question 14
Identify the number of significant digits in the following number:
4090.
Question 15
Identify the number of significant digits in the following number:
5020.
Question Group 6
Question 16
Identify the number of significant digits in the following number:
0.00350
Question 17
Identify the number of significant digits in the following number:
0.00490
Question 18
Identify the number of significant digits in the following number:
0.00760
Activity 2: Measurement
Question Group 7
 Question 19
Question 19
A Chemistry student is measuring the volume of water using a graduated cylinder. A picture of the water level in the cylinder is shown. What is the proper means of reporting the measurement of the volume of water?
16 mL
16.0 mL
16.6 mL
16.60 mL
17.0 mL
17 mL
Question 20
 A Chemistry student is measuring the volume of water using a graduated cylinder. A picture of the water level in the cylinder is shown. What is the proper means of reporting the measurement of the volume of water?
A Chemistry student is measuring the volume of water using a graduated cylinder. A picture of the water level in the cylinder is shown. What is the proper means of reporting the measurement of the volume of water?
23 mL
23.0 mL
23.2 mL
23.20 mL
24.0 mL
24 mL
Question 21
 A Chemistry student is measuring the volume of water using a graduated cylinder. A picture of the water level in the cylinder is shown. What is the proper means of reporting the measurement of the volume of water?
A Chemistry student is measuring the volume of water using a graduated cylinder. A picture of the water level in the cylinder is shown. What is the proper means of reporting the measurement of the volume of water?
37 mL
37.0 mL
37.7 mL
37.70 mL
38.0 mL
38 mL
Question Group 8
Question 22
A Chemistry student is measuring the volume of water using a graduated cylinder. A picture of the water level in the cylinder is shown. What is the proper means of reporting the measurement of the volume of water?
13 mL
13.0 mL
14 mL
14.0 mL
14.00 mL
15 mL
Question 23
 A Chemistry student is measuring the volume of water using a graduated cylinder. A picture of the water level in the cylinder is shown. What is the proper means of reporting the measurement of the volume of water?
A Chemistry student is measuring the volume of water using a graduated cylinder. A picture of the water level in the cylinder is shown. What is the proper means of reporting the measurement of the volume of water?
21 mL
21.0 mL
22 mL
22.0 mL
22.00 mL
23 mL
Question 24
 A Chemistry student is measuring the volume of water using a graduated cylinder. A picture of the water level in the cylinder is shown. What is the proper means of reporting the measurement of the volume of water?
A Chemistry student is measuring the volume of water using a graduated cylinder. A picture of the water level in the cylinder is shown. What is the proper means of reporting the measurement of the volume of water?
35 mL
35.0 mL
36 mL
36.0 mL
36.00 mL
37.0 mL
Question Group 9
Question 25
 A Chemistry student is measuring the volume of water using a graduated cylinder. A picture of the water level in the cylinder is shown. What is the proper means of reporting the measurement of the volume of water?
A Chemistry student is measuring the volume of water using a graduated cylinder. A picture of the water level in the cylinder is shown. What is the proper means of reporting the measurement of the volume of water?
12 mL
12.0 mL
12.4 mL
12.45 mL
13.0 mL
13 mL
Question 26
 A Chemistry student is measuring the volume of water using a graduated cylinder. A picture of the water level in the cylinder is shown. What is the proper means of reporting the measurement of the volume of water?
A Chemistry student is measuring the volume of water using a graduated cylinder. A picture of the water level in the cylinder is shown. What is the proper means of reporting the measurement of the volume of water?
28 mL
28.0 mL
28.6 mL
28.65 mL
29.0 mL
29 mL
Question 27
 A Chemistry student is measuring the volume of water using a graduated cylinder. A picture of the water level in the cylinder is shown. What is the proper means of reporting the measurement of the volume of water?
A Chemistry student is measuring the volume of water using a graduated cylinder. A picture of the water level in the cylinder is shown. What is the proper means of reporting the measurement of the volume of water?
34 mL
34.0 mL
34.2 mL
34.25 mL
35.0 mL
35 mL
Question Group 10
Question 28
Anna Litical is measuring the length of a magnesium strip using a centimeter ruler. She carefully places one end of the magnesium strip at the 0.00-cm mark and aligns the rest of the strip parallel to the ruler. The opposite end of the strip is shown. What is the proper means of reporting the measurement of the length of the magnesium strip?

14 cm
14.5 cm
14.7 cm
14.70 cm
15.0 cm
15 cm
Question 29
Anna Litical is measuring the length of a magnesium strip using a centimeter ruler. She carefully places one end of the magnesium strip at the 0.00-cm mark and aligns the rest of the strip parallel to the ruler. The opposite end of the strip is shown. What is the proper means of reporting the measurement of the length of the magnesium strip?

15 cm
15.5 cm
15.9 cm
15.90 cm
16.0 cm
16 cm
Question 30
Anna Litical is measuring the length of a magnesium strip using a centimeter ruler. She carefully places one end of the magnesium strip at the 0.00-cm mark and aligns the rest of the strip parallel to the ruler. The opposite end of the strip is shown. What is the proper means of reporting the measurement of the length of the magnesium strip?

22 cm
22.3 cm
22.30 cm
22.5 cm
23.0 cm
23 cm
Question Group 11
Question 31
Anna Litical is measuring the length of a magnesium strip using a centimeter ruler. She carefully places one end of the magnesium strip at the 0.00-cm mark and aligns the rest of the strip parallel to the ruler. The opposite end of the strip is shown. What is the proper means of reporting the measurement of the length of the magnesium strip?

13 cm
13.0 cm
14 cm
14.0 cm
14.00 cm
15 cm
Question 32
Anna Litical is measuring the length of a magnesium strip using a centimeter ruler. She carefully places one end of the magnesium strip at the 0.00-cm mark and aligns the rest of the strip parallel to the ruler. The opposite end of the strip is shown. What is the proper means of reporting the measurement of the length of the magnesium strip?

15 cm
15.0 cm
15.00 cm
16.0 cm
16 cm
18 cm
Question 33
Anna Litical is measuring the length of a magnesium strip using a centimeter ruler. She carefully places one end of the magnesium strip at the 0.00-cm mark and aligns the rest of the strip parallel to the ruler. The opposite end of the strip is shown. What is the proper means of reporting the measurement of the length of the magnesium strip?

21 cm
22 cm
22.0 cm
22.00 cm
23.0 cm
23 cm
Question Group 12
Question 34
Anna Litical is measuring the length of a magnesium strip using a centimeter ruler. She carefully places one end of the magnesium strip at the 0.00-cm mark and aligns the rest of the strip parallel to the ruler. The opposite end of the strip is shown. What is the proper means of reporting the measurement of the length of the magnesium strip?
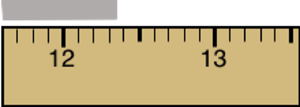
12 cm
12.3 cm
12.35 cm
12.5 cm
13.0 cm
13 cm
Question 35
Anna Litical is measuring the length of a magnesium strip using a centimeter ruler. She carefully places one end of the magnesium strip at the 0.00-cm mark and aligns the rest of the strip parallel to the ruler. The opposite end of the strip is shown. What is the proper means of reporting the measurement of the length of the magnesium strip?

17 cm
17.5 cm
17.7 cm
17.75 cm
18.0 cm
18 cm
Question 36
Anna Litical is measuring the length of a magnesium strip using a centimeter ruler. She carefully places one end of the magnesium strip at the 0.00-cm mark and aligns the rest of the strip parallel to the ruler. The opposite end of the strip is shown. What is the proper means of reporting the measurement of the length of the magnesium strip?

24 cm
24.5 cm
24.8 cm
24.85 cm
25.0 cm
25 cm
Activity 3: Mathematical Operations
Question Group 13
Question 37
 A lab group is attempting to determine the volume of some zinc beads. So they fill a graduated cylinder to the 42.55-mL mark with water. They carefully add the beads to the cylinder. They observe that the water level rises to 76.85 mL. What value should they report as the volume of the zinc beads?
A lab group is attempting to determine the volume of some zinc beads. So they fill a graduated cylinder to the 42.55-mL mark with water. They carefully add the beads to the cylinder. They observe that the water level rises to 76.85 mL. What value should they report as the volume of the zinc beads?
34.30 mL
34.3 mL
119.40 mL
119.4 mL
59.7 mL
59.70 mL
Question 38
 A lab group is attempting to determine the volume of some zinc beads. So they fill a graduated cylinder to the 25.45-mL mark with water. They carefully add the beads to the cylinder. They observe that the water level rises to 46.65 mL. What value should they report as the volume of the zinc beads?
A lab group is attempting to determine the volume of some zinc beads. So they fill a graduated cylinder to the 25.45-mL mark with water. They carefully add the beads to the cylinder. They observe that the water level rises to 46.65 mL. What value should they report as the volume of the zinc beads?
21.20 mL
21.2 mL
72.10 mL
72.1 mL
36.05 mL
36.0 mL
Question 39
 A lab group is attempting to determine the volume of some zinc beads. So they fill a graduated cylinder to the 34.55-mL mark with water. They carefully add the beads to the cylinder. They observe that the water level rises to 66.55 mL. What value should they report as the volume of the zinc beads?
A lab group is attempting to determine the volume of some zinc beads. So they fill a graduated cylinder to the 34.55-mL mark with water. They carefully add the beads to the cylinder. They observe that the water level rises to 66.55 mL. What value should they report as the volume of the zinc beads?
32.20 mL
32.2 mL
101.3 mL
101.30 mL
50.65 mL
50.6 mL
Question Group 14
 Question 40
Question 40
Kara Fulmezzurer is attempting to determine the mass of some aluminum beads. Kara places a dry beaker on a digital balance and determines its mass to be 122.43 grams. She carefully adds the aluminum beads to the same beaker. She determines that the mass of the beaker with the beads is 158.63 grams. What value should Kara report as the mass of the aluminum beads?
36.20 g
36.2 g
281.06 g
281.0 g
140.53 g
140.5 g
Question 41
 Kara Fulmezzurer is attempting to determine the mass of some aluminum beads. Kara places a dry beaker on a digital balance and determines its mass to be 113.52 grams. She carefully adds the aluminum beads to the same beaker. She determines that the mass of the beaker with the beads is 149.72 grams. What value should Kara report as the mass of the aluminum beads?
Kara Fulmezzurer is attempting to determine the mass of some aluminum beads. Kara places a dry beaker on a digital balance and determines its mass to be 113.52 grams. She carefully adds the aluminum beads to the same beaker. She determines that the mass of the beaker with the beads is 149.72 grams. What value should Kara report as the mass of the aluminum beads?
36.20 g
36.2 g
263.24 g
263.2 g
131.62 g
131.6 g
Question 42
 Kara Fulmezzurer is attempting to determine the mass of some aluminum beads. Kara places a dry beaker on a digital balance and determines its mass to be 132.58 grams. She carefully adds the aluminum beads to the same beaker. She determines that the mass of the beaker with the beads is 164.98 grams. What value should Kara report as the mass of the aluminum beads?
Kara Fulmezzurer is attempting to determine the mass of some aluminum beads. Kara places a dry beaker on a digital balance and determines its mass to be 132.58 grams. She carefully adds the aluminum beads to the same beaker. She determines that the mass of the beaker with the beads is 164.98 grams. What value should Kara report as the mass of the aluminum beads?
32.40 g
32.4 g
297.56 g
297.5 g
148.78 g
148.7 g
Question Group 15
Question 43
 Ty Trashun is adding acid from a buret to a flask. He needs to know the volume of acid solution that he adds. So Ty measures the initial volume reading of acid solution in the buret to be 2.45 mL. He measures the final volume reading to be 18.65 mL. What value should Ty report as the volume of acid added to the flask?
Ty Trashun is adding acid from a buret to a flask. He needs to know the volume of acid solution that he adds. So Ty measures the initial volume reading of acid solution in the buret to be 2.45 mL. He measures the final volume reading to be 18.65 mL. What value should Ty report as the volume of acid added to the flask?
16.20 mL
16.2 mL
21.10 mL
21.1 mL
10.55 mL
10.5 mL
Question 44
 Ty Trashun is adding acid from a buret to a flask. He needs to know the volume of acid solution that he adds. So Ty measures the initial volume reading of acid solution in the buret to be 1.37 mL. He measures the final volume reading to be 22.57 mL. What value should Ty report as the volume of acid added to the flask?
Ty Trashun is adding acid from a buret to a flask. He needs to know the volume of acid solution that he adds. So Ty measures the initial volume reading of acid solution in the buret to be 1.37 mL. He measures the final volume reading to be 22.57 mL. What value should Ty report as the volume of acid added to the flask?
21.20 mL
21.2 mL
23.94 mL
23.9 mL
11.97 mL
11.9 mL
Question 45
 Ty Trashun is adding acid from a buret to a flask. He needs to know the volume of acid solution that he adds. So Ty measures the initial volume reading of acid solution in the buret to be 4.36 mL. He measures the final volume reading to be 26.56 mL. What value should Ty report as the volume of acid added to the flask?
Ty Trashun is adding acid from a buret to a flask. He needs to know the volume of acid solution that he adds. So Ty measures the initial volume reading of acid solution in the buret to be 4.36 mL. He measures the final volume reading to be 26.56 mL. What value should Ty report as the volume of acid added to the flask?
22.20 mL
22.2 mL
30.92 mL
30.9 mL
15.46 mL
15.4 mL