pH and pOH - Questions
The pH and pOH Concept Builder is comprised of 12 questions. The questions are divided into three different difficulty levels. The Questions involve tables of numerical data associated with pH, pOH and hydronium and hydroxide ion concentration. There are either missing table cells (10 for
Apprentice Difficulty Level, 18 for
Master Difficulty Level, and 28 for
Wizard Difficulty Level). The Concept Builder selects at random one of the tables for each difficulty level. Students must use the given information to determine the numerical values for the missing table cells. The number of misses are tracked and used to determine a Health Percentage. The Health Percentage is reported once the Table is complete. Students have the option of repeating the Difficulty Level in order to improve their Health Percentage.
The 12 different tables are shown below. Teachers are encouraged to view the tables in order to judge which level of difficulty is most appropriate for their classes. The
Apprentice Difficulty Level is quicker and easier. The
Master and the
Wizard Difficulty Level are of comparable difficulty.
The Physics Classroom grants teachers and other users the right to print these questions for private use. Users are also granted the right to copy the text and modify it for their own use. However, this document should not be uploaded to other servers for distribution to and/or display by others. The Physics Classroom website should remain the only website or server from which the document is distributed or displayed. We also provide a PDF that teachers can use under the same conditions. We have included a link to the PDF near the bottom of this page.
pH-pOH Relationships
Activity 1: Apprentice Difficulty Level
Question 1:
Express your understanding of pH, pOH, and the identity of a solution as being acidic, basic, or neutral by completing the table.
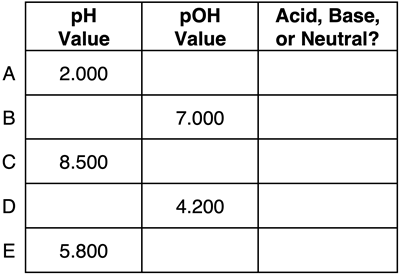
Question 2:
Express your understanding of pH, pOH, ion concentrations, and the identity of a solution as being acidic, basic, or neutral by completing the table.

Question 3:
Express your understanding of pH, pOH, and the identity of a solution as being acidic, basic, or neutral by completing the table.
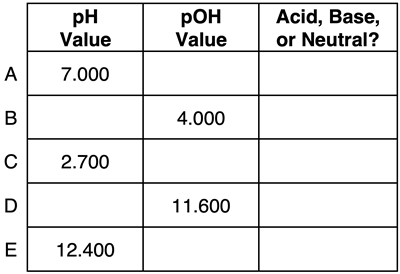
Question 4:
Express your understanding of pH, pOH, and the identity of a solution as being acidic, basic, or neutral by completing the table.
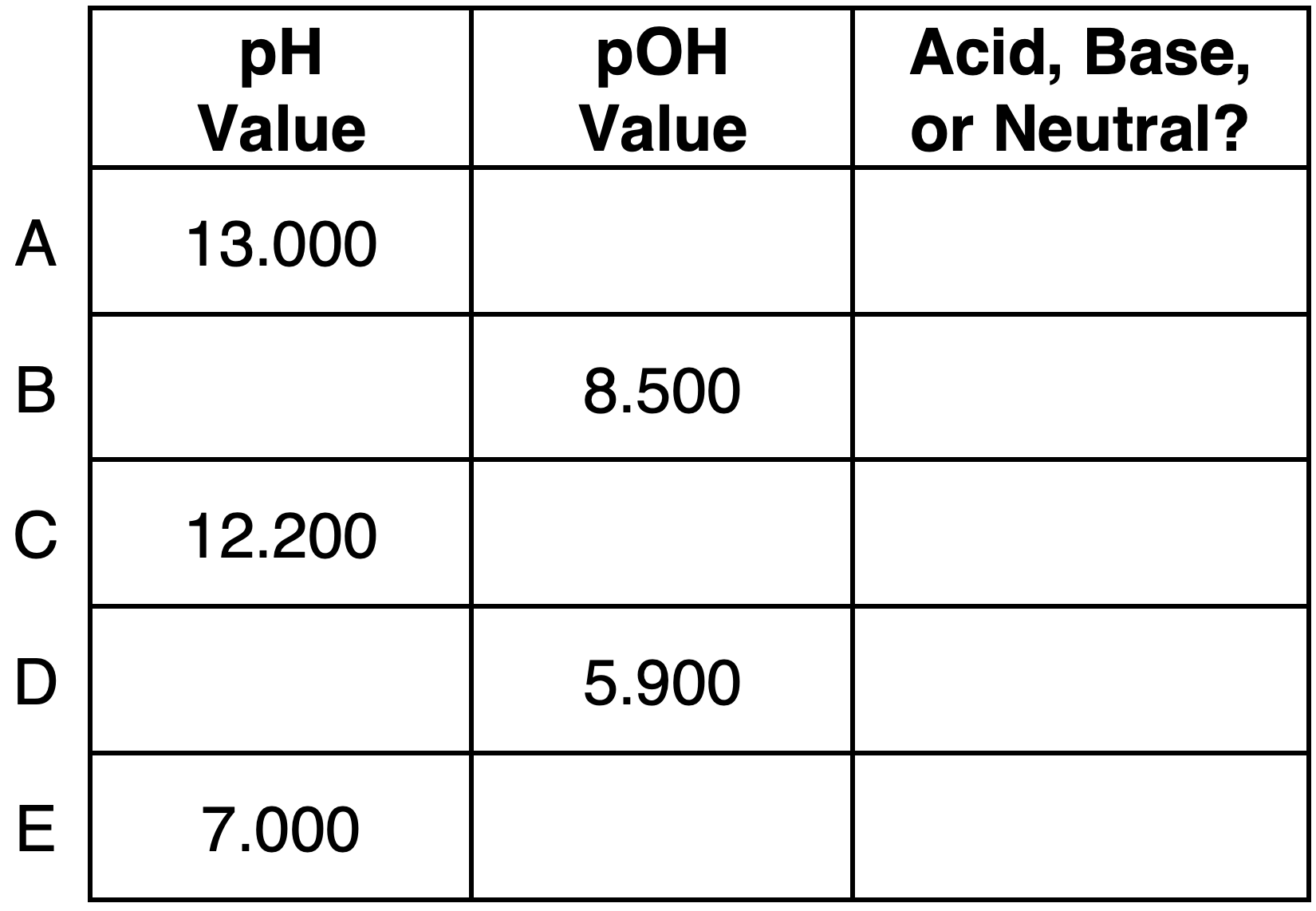
Activity 2: Master Difficulty Level
Question 5:
Express your understanding of pH, pOH, ion concentrations, and the identity of a solution as being acidic, basic, or neutral by completing the table.

Question 6:
Express your understanding of pH, pOH, ion concentrations, and the identity of a solution as being acidic, basic, or neutral by completing the table.
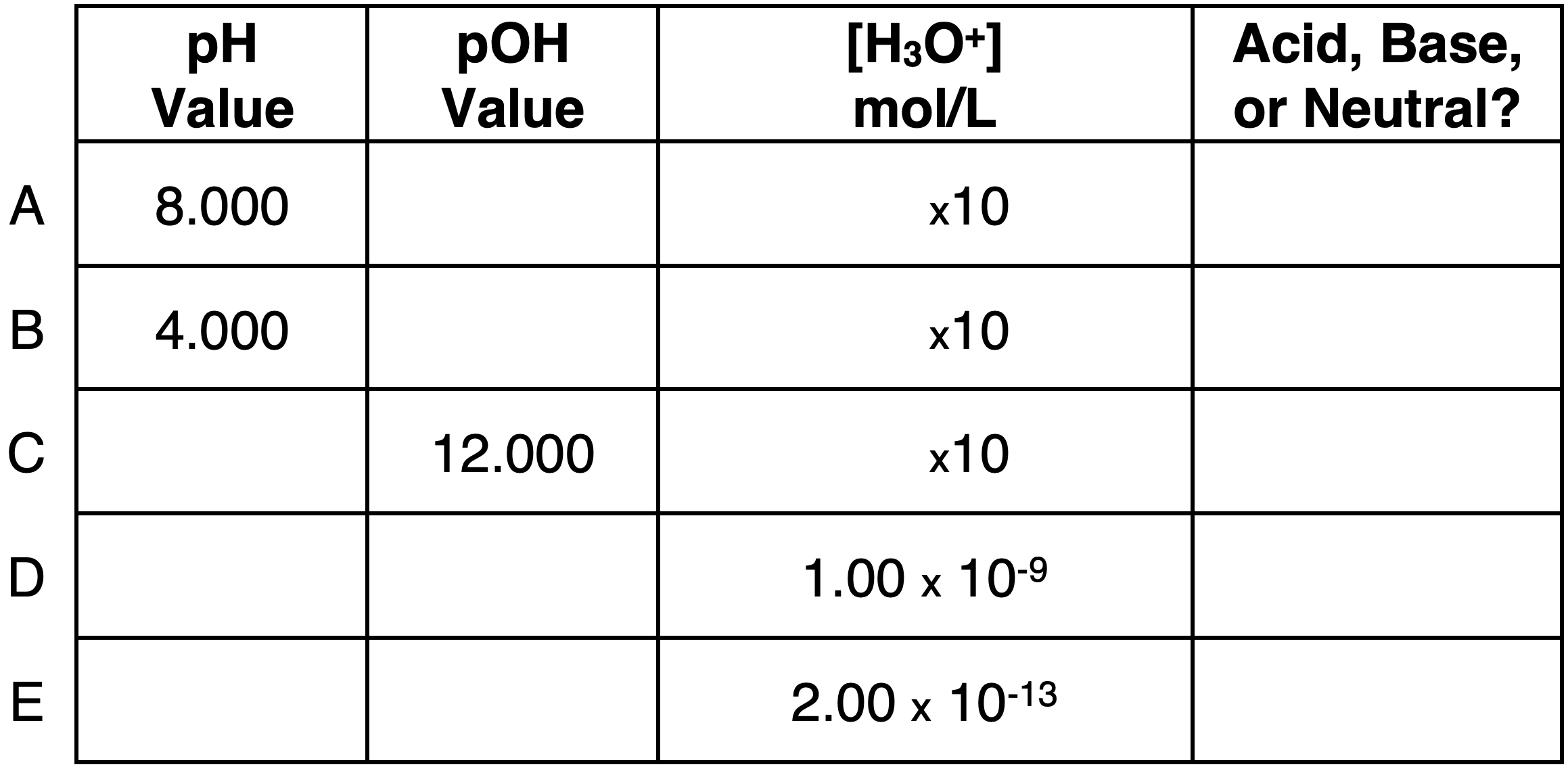
Question 7:
Express your understanding of pH, pOH, ion concentrations, and the identity of a solution as being acidic, basic, or neutral by completing the table.

Question 8:
Express your understanding of pH, pOH, ion concentrations, and the identity of a solution as being acidic, basic, or neutral by completing the table.
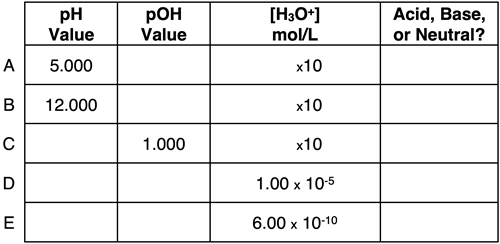
Activity 3: Wizard Difficulty Level
Question 9:
Express your understanding of pH, pOH, ion concentrations, and the identity of a solution as being acidic, basic, or neutral by completing the table.
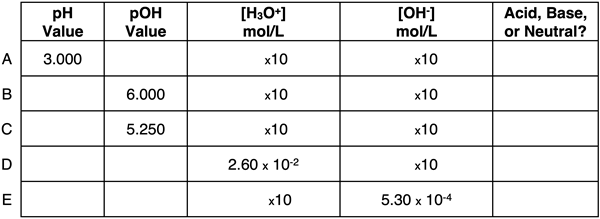
Question 10:
Express your understanding of pH, pOH, ion concentrations, and the identity of a solution as being acidic, basic, or neutral by completing the table.
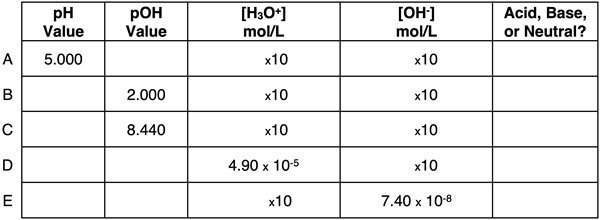
Question 11:
Express your understanding of pH, pOH, ion concentrations, and the identity of a solution as being acidic, basic, or neutral by completing the table.
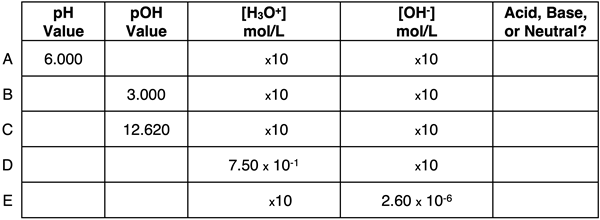
Question 12:
Express your understanding of pH, pOH, ion concentrations, and the identity of a solution as being acidic, basic, or neutral by completing the table.
