Lab Procedures - Questions
The Lab Procedures Concept Builder is comprised of 68 questions. The questions are organized into 17 different Question Groups and spread across four activities. Questions in the same group are rather similar to one another; they may include the same formulas with differing coefficients. The Concept Builder is coded to select at random a question from each group until a student is successful with that group of questions.
There are four activities that can be engaged in through the Concept Builder. Those four activities are differentiated as follows:
- Using a Mass Balance: Question Groups 1-4 ... Questions focus on how to use a Tare button and a weigh boat and the importance of reporting trailing zeroes.
- Using a Bunsen Burner: Question Groups 5-10 ... Questions focus on how to light a burner and how to adjust the air vents; attention is given to the importance of flame color and shape and how to adjust it and identifying the hottest location in the flame.
- Measuremnt: Question Groups 11-14 ... Questions target the importance of sighting head-on, reading from the bottom of a meniscus, and the importance of the estimated digit in a measurement.
- From Bottle to Beaker: Question Groups 15-17 ... Questions focus on how to mix acid and water and capping a bottle after use.
The questions from each group are shown below. Teachers are encouraged to view the questions in order to judge which activities are most appropriate for their classes. We recommend all three activities. The activities are independent of one another.
The Physics Classroom grants teachers and other users the right to print these questions for private use. Users are also granted the right to copy the text and modify it for their own use. However, this document should not be uploaded to other servers for distribution to and/or display by others. The Physics Classroom website should remain the only website or server from which the document is distributed or displayed. We also provide a PDF that teachers can use under the same conditions. We have included a link to the PDF near the bottom of this page.
Lab Procedures
Activity 1: Using a Mass Balance
Question Group 1
Question 1
A student places an empty beaker on the electronic balance. The LCD display reads 265.20 grams. The student records “Beaker Mass = 265.2 g” in his lab notebook. Is this acceptable or unacceptable and why?
A. The zero should not be dropped since it reveals information about the degree of precision to which the mass is known.
B. Dropping the zero at the end is acceptable since it doesn’t make a difference to the amount.
C. Dropping the zero is inappropriate because it makes the teacher mad, something that one should always consider.
D. Dropping the trailing zero is acceptable since it is not a significant digit; it’s just an estimated digit.
Question 2
A student places an empty beaker on the electronic balance. The LCD display reads 265.20 grams. The student records “Beaker Mass = 265.2 g” in his lab notebook. Is this acceptable or unacceptable and why?
A. Dropping the zero at the end is acceptable since it doesn’t make a difference to the amount.
B. Dropping the zero is inappropriate because it makes the teacher mad, something that one should always consider.
C. Dropping the trailing zero is acceptable since it is not a significant digit; it’s just an estimated digit.
D. The zero should not be dropped since it reveals information about the degree of precision to which the mass is known.
Question 3
A student places an empty beaker on the electronic balance. The LCD display reads 265.20 grams. The student records “Beaker Mass = 265.2 g” in his lab notebook. Is this acceptable or unacceptable and why?
A. Dropping the zero is inappropriate because it makes the teacher mad, something that one should always consider.
B. Dropping the trailing zero is acceptable since it is not a significant digit; it’s just an estimated digit.
C. The zero should not be dropped since it reveals information about the degree of precision to which the mass is known.
D. Dropping the zero at the end is acceptable since it doesn’t make a difference to the amount.
Question 4
A student places an empty beaker on the electronic balance. The LCD display reads 265.20 grams. The student records “Beaker Mass = 265.2 g” in his lab notebook. Is this acceptable or unacceptable and why?
A. Dropping the zero at the end is acceptable since it doesn’t make a difference to the amount.
B. Dropping the zero is inappropriate because it makes the teacher mad, something that one should always consider.
C. Dropping the trailing zero is acceptable since it is not a significant digit; it’s just an estimated digit.
D. The zero should not be dropped since it reveals information about the degree of precision to which the mass is known.
Question Group 2
Question 5
Anna Litical is preparing to measure out a certain mass of NaOH pellets using an electronic balance. Which one of the following considerations is most important?
A. Anna should place a weigh boat on the balance and tare it. Then she should scoop the NaOH pellets out of the bottle into the boat
B. Anna should scoop the NaOH pellets onto the balance; she should momentarily remove her goggles to get a clear mass reading.
C. Anna should place the NaOH pellets directly on the balance in order to obtain the most accurate reading.
D. Anna should pour some NaOH onto the lab table and add pellets one at a time until she has what she needs. The excess pellets should be returned to the bottle.
Question 6
Anna Litical is preparing to measure out a certain mass of NaOH pellets using an electronic balance. Which one of the following considerations is most important?
A. Anna should scoop the NaOH pellets onto the balance; she should momentarily remove her goggles to get a clear mass reading.
B. Anna should place the NaOH pellets directly on the balance in order to obtain the most accurate reading.
C. Anna should pour some NaOH onto the lab table and add pellets one at a time until she has what she needs. The excess pellets should be returned to the bottle.
D. Anna should place a weigh boat on the balance and tare it. Then she should scoop the NaOH pellets out of the bottle into the boat
Question 7
Anna Litical is preparing to measure out a certain mass of NaOH pellets using an electronic balance. Which one of the following considerations is most important?
A. Anna should place the NaOH pellets directly on the balance in order to obtain the most accurate reading.
B. Anna should pour some NaOH onto the lab table and add pellets one at a time until she has what she needs. The excess pellets should be returned to the bottle.
C. Anna should place a weigh boat on the balance and tare it. Then she should scoop the NaOH pellets out of the bottle into the boat
D. Anna should scoop the NaOH pellets onto the balance; she should momentarily remove her goggles to get a clear mass reading.
Question 8
Anna Litical is preparing to measure out a certain mass of NaOH pellets using an electronic balance. Which one of the following considerations is most important?
A. Anna should pour some NaOH onto the lab table and add pellets one at a time until she has what she needs. The excess pellets should be returned to the bottle.
B. Anna should place a weigh boat on the balance and tare it. Then she should scoop the NaOH pellets out of the bottle into the boat
C. Anna should scoop the NaOH pellets onto the balance; she should momentarily remove her goggles to get a clear mass reading.
D. Anna should place the NaOH pellets directly on the balance in order to obtain the most accurate reading.
Question Group 3
Question 9
During the
Dense Cents lab, Noah Formula places a 3.24-gram weigh boat on the electronic balance and tares it. Noah then adds 20 pennies to the weigh boat. The electronic balance reads 62.56 gram. What expression gives the mass of the 20 pennies (in grams)?
A. 62.56
B. 62.56 – 3.24
C. 20*(62.56 – 3.24)
D. 20*3.24 – 62.56
Question 10
During the
Dense Cents lab, Noah Formula places a 3.24-gram weigh boat on the electronic balance and tares it. Noah then adds 20 pennies to the weigh boat. The electronic balance reads 62.56 gram. What expression gives the mass of the 20 pennies (in grams)?
A. 62.56 – 3.24
B. 62.56
C. 20*3.24 – 62.56
D. 20*(62.56 – 3.24)
Question 11
During the
Dense Cents lab, Noah Formula places a 3.24-gram weigh boat on the electronic balance and tares it. Noah then adds 20 pennies to the weigh boat. The electronic balance reads 62.56 gram. What expression gives the mass of the 20 pennies (in grams)?
A. 20*(62.56 – 3.24)
B. 20*3.24 – 62.56
C. 62.56
D. 62.56 – 3.24
Question 12
During the
Dense Cents lab, Noah Formula places a 3.24-gram weigh boat on the electronic balance and tares it. Noah then adds 20 pennies to the weigh boat. The electronic balance reads 62.56 gram. What expression gives the mass of the 20 pennies (in grams)?
A. 20*3.24 – 62.56
B. 20*(62.56 – 3.24)
C. 62.56 – 3.24
D. 62.56
Question Group 4
Question 13
During the
Dense Cents lab, Noah Formula places a weigh boat on the electronic balance; the balance reads 3.05 gram. Noah then adds 20 pennies to the weigh boat. The electronic balance reads 54.08 gram. What expression gives the mass of the 20 pennies (in grams)?
A. 54.08
B. 54.08 – 3.05
C. (54.08 – 3.05)/20
D. (54.08 – 20)*3.05
Question 14
During the
Dense Cents lab, Noah Formula places a weigh boat on the electronic balance; the balance reads 3.05 gram. Noah then adds 20 pennies to the weigh boat. The electronic balance reads 54.08 gram. What expression gives the mass of the 20 pennies (in grams)?
A. 54.08 – 3.05
B. 54.08
C. (54.08 – 20)*3.05
D. (54.08 – 3.05)/20
Question 15
During the
Dense Cents lab, Noah Formula places a weigh boat on the electronic balance; the balance reads 3.05 gram. Noah then adds 20 pennies to the weigh boat. The electronic balance reads 54.08 gram. What expression gives the mass of the 20 pennies (in grams)?
A. (54.08 – 3.05)/20
B. (54.08 – 20)*3.05
C. 54.08
D. 54.08 – 3.05
Question 16
During the
Dense Cents lab, Noah Formula places a weigh boat on the electronic balance; the balance reads 3.05 gram. Noah then adds 20 pennies to the weigh boat. The electronic balance reads 54.08 gram. What expression gives the mass of the 20 pennies (in grams)?
A. (54.08 – 20)*3.05
B. (54.08 – 3.05)/20
C. 54.08 – 3.05
D. 54.08
Activity 2: Using a Bunsen Burner
Question Group 5
Question 17
Suppose you must light a Bunsen burner with a striker (or lighter). The gas is exiting the burner and now you must light it. What is the best way to use the striker?
A. Hold the striker 1-2 inches above the burner opening. Squeeze the striker to make a spark.
B. From a safe distance away, squeeze the striker while using a flicking motion to direct a spark towards the burner.
C. Hold the striker just below the burner opening. Squeeze the striker to make a spark.
D. Hold the striker a meter above the burner. Squeeze the striker as you make a rapid downward motion towards the burner.
Question 18
Suppose you must light a Bunsen burner with a striker (or lighter). The gas is exiting the burner and now you must light it. What is the best way to use the striker?
A. From a safe distance away, squeeze the striker while using a flicking motion to direct a spark towards the burner.
B. Hold the striker just below the burner opening. Squeeze the striker to make a spark.
C. Hold the striker a meter above the burner. Squeeze the striker as you make a rapid downward motion towards the burner.
D. Hold the striker 1-2 inches above the burner opening. Squeeze the striker to make a spark.
Question 19
Suppose you must light a Bunsen burner with a striker (or lighter). The gas is exiting the burner and now you must light it. What is the best way to use the striker?
A. Hold the striker just below the burner opening. Squeeze the striker to make a spark.
B. Hold the striker a meter above the burner. Squeeze the striker as you make a rapid downward motion towards the burner.
C. Hold the striker 1-2 inches above the burner opening. Squeeze the striker to make a spark.
D. From a safe distance away, squeeze the striker while using a flicking motion to direct a spark towards the burner.
Question 20
Suppose you must light a Bunsen burner with a striker (or lighter). The gas is exiting the burner and now you must light it. What is the best way to use the striker?
A. Hold the striker a meter above the burner. Squeeze the striker as you make a rapid downward motion towards the burner.
B. Hold the striker 1-2 inches above the burner opening. Squeeze the striker to make a spark.
C. From a safe distance away, squeeze the striker while using a flicking motion to direct a spark towards the burner.
D. Hold the striker just below the burner opening. Squeeze the striker to make a spark.
Question Group 6
Question 21
A Bunsen burner is equipped with air vents. A metal collar surrounding the barrel can be turned to adjust the size of the air vent openings. Before lighting a Bunsen burner, it is best to ________
A. Adjust the metal collar so that the vents are closed or mostly closed.
B. Adjust the metal collar so that the vents are fully open.
C. Adjust the metal collar so that the vents are halfway open.
D. Adjust the metal collar so that half of the vents are open and the other half are closed.
Question 22
A Bunsen burner is equipped with air vents. A metal collar surrounding the barrel can be turned to adjust the size of the air vent openings. Before lighting a Bunsen burner, it is best to ________
A. Adjust the metal collar so that the vents are fully open.
B. Adjust the metal collar so that the vents are halfway open.
C. Adjust the metal collar so that half of the vents are open and the other half are closed.
D. Adjust the metal collar so that the vents are closed or mostly closed.
Question 23
A Bunsen burner is equipped with air vents. A metal collar surrounding the barrel can be turned to adjust the size of the air vent openings. Before lighting a Bunsen burner, it is best to ________
A. Adjust the metal collar so that the vents are halfway open.
B. Adjust the metal collar so that half of the vents are open and the other half are closed.
C. Adjust the metal collar so that the vents are closed or mostly closed.
D. Adjust the metal collar so that the vents are fully open.
Question 24
A Bunsen burner is equipped with air vents. A metal collar surrounding the barrel can be turned to adjust the size of the air vent openings. Before lighting a Bunsen burner, it is best to ________
A. Adjust the metal collar so that half of the vents are open and the other half are closed.
B. Adjust the metal collar so that the vents are closed or mostly closed.
C. Adjust the metal collar so that the vents are fully open.
D. Adjust the metal collar so that the vents are halfway open.
Question Group 7
Question 25
Not all Bunsen burner flames are equal. For best operation, a Bunsen burner should be adjusted so that the flame is _______.
A. a bluish-colored flame with three recognizable cones
B. a bright yellow, candle-like flame
C. a red-hot flame that waves back and forth like a flag
D. as tall as you can get it with an orange tint to it
Question 26
Not all Bunsen burner flames are equal. For best operation, a Bunsen burner should be adjusted so that the flame is _______.
A. a bright yellow, candle-like flame
B. a red-hot flame that waves back and forth like a flag
C. as tall as you can get it with an orange tint to it
D. a bluish-colored flame with three recognizable cones
Question 27
Not all Bunsen burner flames are equal. For best operation, a Bunsen burner should be adjusted so that the flame is _______.
A. a red-hot flame that waves back and forth like a flag
B. as tall as you can get it with an orange tint to it
C. a bluish-colored flame with three recognizable cones
D. a bright yellow, candle-like flame
Question 28
Not all Bunsen burner flames are equal. For best operation, a Bunsen burner should be adjusted so that the flame is _______.
A. as tall as you can get it with an orange tint to it
B. a bluish-colored flame with three recognizable cones
C. a bright yellow, candle-like flame
D. a red-hot flame that waves back and forth like a flag
Question Group 8
Question 29
A lit Bunsen burner has a yellow flame. What is wrong with a yellow flame and what adjustments should be made to it?
A. Yellow flames are not hot enough. Open the air vents slowly until you seen a blue cone-shaped flame.
B. Yellow flames are too hot to use. Open the air vents slowly until you seen a blue cone-shaped flame.
C. Yellow flames are safety hazard. Turn the gas inlet valve to reduce the amount of gas. It will eventually turn a safer yellow.
D. Yellow flames are dangerously hot. The gas should be immediately shut off. Then start over.
Question 30
A lit Bunsen burner has a yellow flame. What is wrong with a yellow flame and what adjustments should be made to it?
A. Yellow flames are too hot to use. Open the air vents slowly until you seen a blue cone-shaped flame.
B. Yellow flames are safety hazard. Turn the gas inlet valve to reduce the amount of gas. It will eventually turn a safer yellow.
C. Yellow flames are dangerously hot. The gas should be immediately shut off. Then start over.
D. Yellow flames are not hot enough. Open the air vents slowly until you seen a blue cone-shaped flame.
Question 31
A lit Bunsen burner has a yellow flame. What is wrong with a yellow flame and what adjustments should be made to it?
A. Yellow flames are safety hazard. Turn the gas inlet valve to reduce the amount of gas. It will eventually turn a safer yellow.
B. Yellow flames are dangerously hot. The gas should be immediately shut off. Then start over.
C. Yellow flames are not hot enough. Open the air vents slowly until you seen a blue cone-shaped flame.
D. Yellow flames are too hot to use. Open the air vents slowly until you seen a blue cone-shaped flame.
Question 32
A lit Bunsen burner has a yellow flame. What is wrong with a yellow flame and what adjustments should be made to it?
A. Yellow flames are dangerously hot. The gas should be immediately shut off. Then start over.
B. Yellow flames are not hot enough. Open the air vents slowly until you seen a blue cone-shaped flame.
C. Yellow flames are too hot to use. Open the air vents slowly until you seen a blue cone-shaped flame.
D. Yellow flames are safety hazard. Turn the gas inlet valve to reduce the amount of gas. It will eventually turn a safer yellow.
Question Group 9
Question 33
The natural gas used by a Bunsen burner is delivered through a supply hose to the burner from the gas outlets located on the lab bench. Before lighting the burner, turn the handle attached to the gas jet so that it is ____________.
A. parallel to the gas outlets and supply hose
B. perpendicular to the gas outlets and supply hose; twisted clockwise to the right
C. perpendicular to the gas outlets and supply hose; twisted counterclockwise to the left
D. at a 45° angle to the gas outlets and supply hose
Question 34
The natural gas used by a Bunsen burner is delivered through a supply hose to the burner from the gas outlets located on the lab bench. Before lighting the burner, turn the handle attached to the gas jet so that it is ____________.
A. perpendicular to the gas outlets and supply hose; twisted clockwise to the right
B. perpendicular to the gas outlets and supply hose; twisted counterclockwise to the left
C. at a 45° angle to the gas outlets and supply hose
D. parallel to the gas outlets and supply hose
Question 35
The natural gas used by a Bunsen burner is delivered through a supply hose to the burner from the gas outlets located on the lab bench. Before lighting the burner, turn the handle attached to the gas jet so that it is ____________.
A. perpendicular to the gas outlets and supply hose; twisted counterclockwise to the left
B. at a 45° angle to the gas outlets and supply hose
C. parallel to the gas outlets and supply hose
D. perpendicular to the gas outlets and supply hose; twisted clockwise to the right
Question 36
The natural gas used by a Bunsen burner is delivered through a supply hose to the burner from the gas outlets located on the lab bench. Before lighting the burner, turn the handle attached to the gas jet so that it is ____________.
A. at a 45° angle to the gas outlets and supply hose
B. parallel to the gas outlets and supply hose
C. perpendicular to the gas outlets and supply hose; twisted clockwise to the right
D. perpendicular to the gas outlets and supply hose; twisted counterclockwise to the left
Question Group 10
 Question 37
Question 37
A student has adjusted a Bunsen burner flame. It looks like the diagram at the right. The lab directions state that a copper wire should be dipped in a solution and then placed at the hottest location of the flame. Which location is the hottest location?
A. Location A
B. Location B
C. Location C
D. Location D
 Question 38
Question 38
A student has adjusted a Bunsen burner flame. It looks like the diagram at the right. The lab directions state that a copper wire should be dipped in a solution and then placed at the hottest location of the flame. Which location is the hottest location?
A. Location A
B. Location B
C. Location C
D. Location D
 Question 39
Question 39
A student has adjusted a Bunsen burner flame. It looks like the diagram at the right. The lab directions state that a copper wire should be dipped in a solution and then placed at the hottest location of the flame. Which location is the hottest location?
A. Location A
B. Location B
C. Location C
D. Location D
 Question 40
Question 40
A student has adjusted a Bunsen burner flame. It looks like the diagram at the right. The lab directions state that a copper wire should be dipped in a solution and then placed at the hottest location of the flame. Which location is the hottest location?
A. Location A
B. Location B
C. Location C
D. Location D
Activity 3: Measuring
Question Group 11
Question 41
Anna Litical is preparing to measure the volume of an acidic solution in a burette. The solution forms a meniscus. How should Anna make her reading?
A. Anna should make her reading based on the bottom of the meniscus.
B. Anna should make her reading based on the top of the meniscus.
C. Anna should make her reading based on the middle of the meniscus.
D. Anna should average the readings for the top and the bottom of the meniscus.
Question 42
Anna Litical is preparing to measure the volume of an acidic solution in a burette. The solution forms a meniscus. How should Anna make her reading?
A. Anna should make her reading based on the top of the meniscus.
B. Anna should make her reading based on the middle of the meniscus.
C. Anna should average the readings for the top and the bottom of the meniscus.
D. Anna should make her reading based on the bottom of the meniscus.
Question 43
Anna Litical is preparing to measure the volume of an acidic solution in a burette. The solution forms a meniscus. How should Anna make her reading?
A. Anna should make her reading based on the middle of the meniscus.
B. Anna should average the readings for the top and the bottom of the meniscus.
C. Anna should make her reading based on the bottom of the meniscus.
D. Anna should make her reading based on the top of the meniscus.
Question 44
Anna Litical is preparing to measure the volume of an acidic solution in a burette. The solution forms a meniscus. How should Anna make her reading?
A. Anna should average the readings for the top and the bottom of the meniscus.
B. Anna should make her reading based on the bottom of the meniscus.
C. Anna should make her reading based on the top of the meniscus.
D. Anna should make her reading based on the middle of the meniscus.
Question Group 12
Question 45
Kara Phool is measuring the length of a strip of magnesium using a centimeter ruler with markings every 0.1 cm along the ruler. What considerations should be made when making the length measurement?
A. Kara should record the length to two decimal places. The digit in the second decimal place is an estimated digit.
B. Kara should be as accurate as possible and not do any guessing. She should report the value to a single decimal place.
C. Kara should make five consecutive measurements of length and average the five measurements.
D. Kara should relax and do her best. Needless worrying over how many decimal places to report is not scientific.
Question 46
Kara Phool is measuring the length of a strip of magnesium using a centimeter ruler with markings every 0.1 cm along the ruler. What considerations should be made when making the length measurement?
A. Kara should be as accurate as possible and not do any guessing. She should report the value to a single decimal place.
B. Kara should make five consecutive measurements of length and average the five measurements.
C. Kara should relax and do her best. Needless worrying over how many decimal places to report is not scientific.
D. Kara should record the length to two decimal places. The digit in the second decimal place is an estimated digit.
Question 47
Kara Phool is measuring the length of a strip of magnesium using a centimeter ruler with markings every 0.1 cm along the ruler. What considerations should be made when making the length measurement?
A. Kara should make five consecutive measurements of length and average the five measurements.
B. Kara should relax and do her best. Needless worrying over how many decimal places to report is not scientific.
C. Kara should record the length to two decimal places. The digit in the second decimal place is an estimated digit.
D. Kara should be as accurate as possible and not do any guessing. She should report the value to a single decimal place.
Question 48
Kara Phool is measuring the length of a strip of magnesium using a centimeter ruler with markings every 0.1 cm along the ruler. What considerations should be made when making the length measurement?
A. Kara should relax and do her best. Needless worrying over how many decimal places to report is not scientific.
B. Kara should record the length to two decimal places. The digit in the second decimal place is an estimated digit.
C. Kara should be as accurate as possible and not do any guessing. She should report the value to a single decimal place.
D. Kara should make five consecutive measurements of length and average the five measurements.
Question Group 13
Question 49
Sigmund Figgs (his friends call him “Sig”) is measuring the volume of water in a graduated cylinder. The cylinder has markings every 0.1 mL. Sig notices that the volume is 21 mL - spot on. What volume should Sig record in his lab notebook?
A. 21 mL
B. 21.0 mL
C. 21.00 mL
D. 21.000 mL
Question 50
Sigmund Figgs (his friends call him “Sig”) is measuring the volume of water in a graduated cylinder. The cylinder has markings every 0.1 mL. Sig notices that the volume is 22 mL - spot on. What volume should Sig record in his lab notebook?
A. 22 mL
B. 22.0 mL
C. 22.00 mL
D. 22.000 mL
Question 51
Sigmund Figgs (his friends call him “Sig”) is measuring the volume of water in a graduated cylinder. The cylinder has markings every 0.1 mL. Sig notices that the volume is 18 mL - spot on. What volume should Sig record in his lab notebook?
A. 18 mL
B. 18.0 mL
C. 18.00 mL
D. 18.000 mL
Question 52
Sigmund Figgs (his friends call him “Sig”) is measuring the volume of water in a graduated cylinder. The cylinder has markings every 0.1 mL. Sig notices that the volume is 17 mL - spot on. What volume should Sig record in his lab notebook?
A. 17 mL
B. 17.0 mL
C. 17.00 mL
D. 17.000 mL
Question Group 14
Question 53
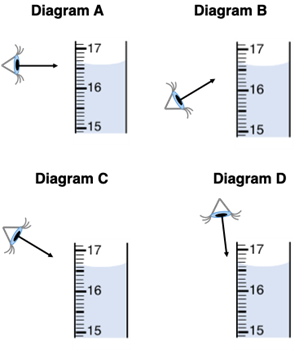
Al Wayskashus is measuring the volume of a solution in a graduated cylinder. In what manner should Al view the cylinder in order to acquire the most accurate reading?
Question 54
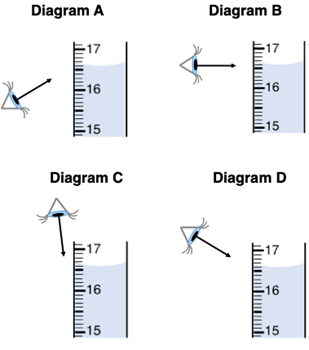
Al Wayskashus is measuring the volume of a solution in a graduated cylinder. In what manner should Al view the cylinder in order to acquire the most accurate reading?
Question 55
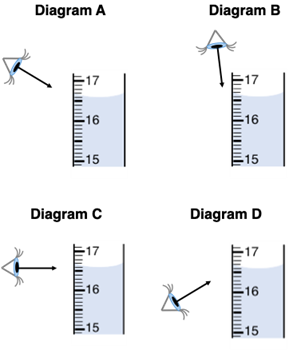
Al Wayskashus is measuring the volume of a solution in a graduated cylinder. In what manner should Al view the cylinder in order to acquire the most accurate reading?
Question 56
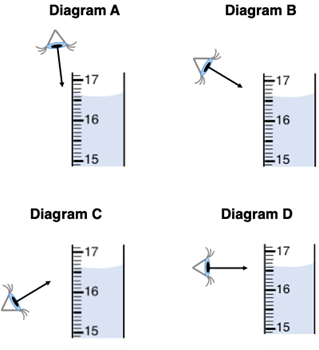
Al Wayskashus is measuring the volume of a solution in a graduated cylinder. In what manner should Al view the cylinder in order to acquire the most accurate reading?
Activity 4: From Bottle to Beaker
Question Group 15
Question 57
A lab procedure step states “Add enough acid to the flask until the solution turns light pink.” The acid is located in a large bottle in a central location that the whole class uses. How should you proceed with this step?
A. Pour some of the acid in a small beaker. Bring it to your lab table. Pour acid from the beaker into the flask until the solution turns pink. Rinse the unused acid down the drain.
B. Pour some of the acid in a small beaker. Bring it to your lab table. Pour acid from the beaker into the flask until the solution turns pink. Return the unused acid to the bottle.
C. Bring the bottle to your lab table. Pour acid from the bottle into the flask until the solution turns pink. You’re guaranteed that there will be no waste with this method.
D. Take your flask to the bottle. Pour acid from the bottle into the flask until the solution turns pink. You’re guaranteed that there will be no waste with this method.
Question 58
A lab procedure step states “Add enough acid to the flask until the solution turns light pink.” The acid is located in a large bottle in a central location that the whole class uses. How should you proceed with this step?
A. Pour some of the acid in a small beaker. Bring it to your lab table. Pour acid from the beaker into the flask until the solution turns pink. Return the unused acid to the bottle.
B. Bring the bottle to your lab table. Pour acid from the bottle into the flask until the solution turns pink. You’re guaranteed that there will be no waste with this method.
C. Take your flask to the bottle. Pour acid from the bottle into the flask until the solution turns pink. You’re guaranteed that there will be no waste with this method.
D. Pour some of the acid in a small beaker. Bring it to your lab table. Pour acid from the beaker into the flask until the solution turns pink. Rinse the unused acid down the drain.
Question 59
A lab procedure step states “Add enough acid to the flask until the solution turns light pink.” The acid is located in a large bottle in a central location that the whole class uses. How should you proceed with this step?
A. Bring the bottle to your lab table. Pour acid from the bottle into the flask until the solution turns pink. You’re guaranteed that there will be no waste with this method.
B. Take your flask to the bottle. Pour acid from the bottle into the flask until the solution turns pink. You’re guaranteed that there will be no waste with this method.
C. Pour some of the acid in a small beaker. Bring it to your lab table. Pour acid from the beaker into the flask until the solution turns pink. Rinse the unused acid down the drain.
D. Pour some of the acid in a small beaker. Bring it to your lab table. Pour acid from the beaker into the flask until the solution turns pink. Return the unused acid to the bottle.
Question 60
A lab procedure step states “Add enough acid to the flask until the solution turns light pink.” The acid is located in a large bottle in a central location that the whole class uses. How should you proceed with this step?
A. Take your flask to the bottle. Pour acid from the bottle into the flask until the solution turns pink. You’re guaranteed that there will be no waste with this method.
B. Pour some of the acid in a small beaker. Bring it to your lab table. Pour acid from the beaker into the flask until the solution turns pink. Rinse the unused acid down the drain.
C. Pour some of the acid in a small beaker. Bring it to your lab table. Pour acid from the beaker into the flask until the solution turns pink. Return the unused acid to the bottle.
D. Bring the bottle to your lab table. Pour acid from the bottle into the flask until the solution turns pink. You’re guaranteed that there will be no waste with this method.
Question Group 16
Question 61
Your lab group has fallen behind and must pick up the pace. The current procedural step requires that you retrieve 25 mL of four different solutions in four labeled beakers at a central filling station. How should you proceed with this step?
A. Take the four beakers to the station. Uncap each bottle and place cap on table. Pour each solution into its beaker. Once done, put all the caps back on the bottles.
B. Take the four beakers to the station. One bottle at a time: uncap the bottle, pour the solution, and recap the bottle. Repeat for all four solutions.
C. Take a beaker to the station. Uncap the bottle and pour the solution. Return to your lab table. Repeat for all four solutions. Once done, be sure to recap all the bottles.
D. Take a large beaker to the station. Uncap all the bottles. Pour the four solutions into the beaker. Recap all the bottles. Return to your lab table and divide the mixture into the beakers.
Question 62
Your lab group has fallen behind and must pick up the pace. The current procedural step requires that you retrieve 25 mL of four different solutions in four labeled beakers at a central filling station. How should you proceed with this step?
A. Take the four beakers to the station. One bottle at a time: uncap the bottle, pour the solution, and recap the bottle. Repeat for all four solutions.
B. Take a beaker to the station. Uncap the bottle and pour the solution. Return to your lab table. Repeat for all four solutions. Once done, be sure to recap all the bottles.
C. Take a large beaker to the station. Uncap all the bottles. Pour the four solutions into the beaker. Recap all the bottles. Return to your lab table and divide the mixture into the beakers.
D. Take the four beakers to the station. Uncap each bottle and place cap on table. Pour each solution into its beaker. Once done, put all the caps back on the bottles.
Question 63
Your lab group has fallen behind and must pick up the pace. The current procedural step requires that you retrieve 25 mL of four different solutions in four labeled beakers at a central filling station. How should you proceed with this step?
A. Take a beaker to the station. Uncap the bottle and pour the solution. Return to your lab table. Repeat for all four solutions. Once done, be sure to recap all the bottles.
B. Take a large beaker to the station. Uncap all the bottles. Pour the four solutions into the beaker. Recap all the bottles. Return to your lab table and divide the mixture into the beakers.
C. Take the four beakers to the station. Uncap each bottle and place cap on table. Pour each solution into its beaker. Once done, put all the caps back on the bottles.
D. Take the four beakers to the station. One bottle at a time: uncap the bottle, pour the solution, and recap the bottle. Repeat for all four solutions.
Question 64
Your lab group has fallen behind and must pick up the pace. The current procedural step requires that you retrieve 25 mL of four different solutions in four labeled beakers at a central filling station. How should you proceed with this step?
A. Take a large beaker to the station. Uncap all the bottles. Pour the four solutions into the beaker. Recap all the bottles. Return to your lab table and divide the mixture into the beakers.
B. Take the four beakers to the station. Uncap each bottle and place cap on table. Pour each solution into its beaker. Once done, put all the caps back on the bottles.
C. Take the four beakers to the station. One bottle at a time: uncap the bottle, pour the solution, and recap the bottle. Repeat for all four solutions.
D. Take a beaker to the station. Uncap the bottle and pour the solution. Return to your lab table. Repeat for all four solutions. Once done, be sure to recap all the bottles.
Question Group 17
Question 65
You’re at the point in a lab procedure where you must dilute 10 mL of a 6 M solution of HCl (an acid) to form approximately 50 mL solution in a flask. You’ve retrieved your 6 M acid in a graduated cylinder. Now you must dilute it at your lab bench. How should you proceed with this step?
A. Pour 40 mL of water in the flask. Tip the flask slightly and slowly add the 10 mL of acid to the water by allowing it to roll down the inside wall of the flask.
B. Acquire 40 mL of water in a second cylinder. Simultaneously pour the water and the acid into the flask so that they mix together upon entry.
C. Pour the 10 mL of acid into the flask. Tip the flask slightly and slowly add the 40 mL of water to the acid by allowing it to roll down the inside wall of the flask.
D. Get a larger cylinder. Pour the acid into the cylinder. Then add water to the cylinder until the solution reaches the 50 mL marking. Pour the cylinder contents into the flask.
Question 66
You’re at the point in a lab procedure where you must dilute 10 mL of a 6 M solution of HCl (an acid) to form approximately 50 mL solution in a flask. You’ve retrieved your 6 M acid in a graduated cylinder. Now you must dilute it at your lab bench. How should you proceed with this step?
A. Acquire 40 mL of water in a second cylinder. Simultaneously pour the water and the acid into the flask so that they mix together upon entry.
B. Pour the 10 mL of acid into the flask. Tip the flask slightly and slowly add the 40 mL of water to the acid by allowing it to roll down the inside wall of the flask.
C. Get a larger cylinder. Pour the acid into the cylinder. Then add water to the cylinder until the solution reaches the 50 mL marking. Pour the cylinder contents into the flask.
D. Pour 40 mL of water in the flask. Tip the flask slightly and slowly add the 10 mL of acid to the water by allowing it to roll down the inside wall of the flask.
Question 67
You’re at the point in a lab procedure where you must dilute 10 mL of a 6 M solution of HCl (an acid) to form approximately 50 mL solution in a flask. You’ve retrieved your 6 M acid in a graduated cylinder. Now you must dilute it at your lab bench. How should you proceed with this step?
A. Pour the 10 mL of acid into the flask. Tip the flask slightly and slowly add the 40 mL of water to the acid by allowing it to roll down the inside wall of the flask.
B. Get a larger cylinder. Pour the acid into the cylinder. Then add water to the cylinder until the solution reaches the 50 mL marking. Pour the cylinder contents into the flask.
C. Pour 40 mL of water in the flask. Tip the flask slightly and slowly add the 10 mL of acid to the water by allowing it to roll down the inside wall of the flask.
D. Acquire 40 mL of water in a second cylinder. Simultaneously pour the water and the acid into the flask so that they mix together upon entry.
Question 68
You’re at the point in a lab procedure where you must dilute 10 mL of a 6 M solution of HCl (an acid) to form approximately 50 mL solution in a flask. You’ve retrieved your 6 M acid in a graduated cylinder. Now you must dilute it at your lab bench. How should you proceed with this step?
A. Get a larger cylinder. Pour the acid into the cylinder. Then add water to the cylinder until the solution reaches the 50 mL marking. Pour the cylinder contents into the flask.
B. Pour 40 mL of water in the flask. Tip the flask slightly and slowly add the 10 mL of acid to the water by allowing it to roll down the inside wall of the flask.
C. Acquire 40 mL of water in a second cylinder. Simultaneously pour the water and the acid into the flask so that they mix together upon entry.
D. Pour the 10 mL of acid into the flask. Tip the flask slightly and slowly add the 40 mL of water to the acid by allowing it to roll down the inside wall of the flask.