Hold down the T key for 3 seconds to activate the audio accessibility mode, at which point you can click the K key to pause and resume audio. Useful for the Check Your Understanding and See Answers.
Lesson 1: Describing Chemical Reactions
Part a: What is a Chemical Reaction?
Part a: What is a Chemical Reaction?
Part b:
Chemical Equations
Part c:
Writing Balanced Chemical Equations
Chemical Change … Revisited
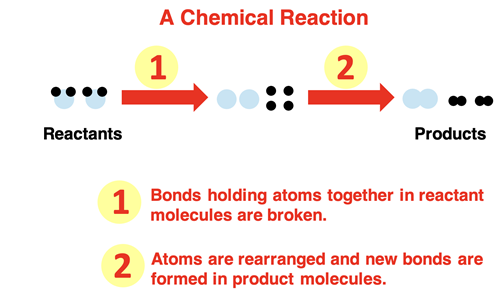
In Chapter 2 of this Chemistry Tutorial, the distinction between physical and chemical change was discussed. A chemical change involves a chemical reaction. In chemical reactions, the bonds that hold atoms together in molecules are broken. The atoms re-arrange and form new bonds, resulting in the production of new chemicals. The reactant chemicals are changed to product chemicals.
 Because the product chemicals are different than the reactant chemicals, changes are commonly observed in the system. The changes that are observed serve as evidence that a chemical reaction has taken or is taking place. A color change is often observed. Bubbles can be observed being formed in the system or an odor that was not previously present might be detected. A solid might be formed at the bottom of a solution (known as a precipitate). On occasion there is a noticeable change in temperature of the surroundings as energy is released from or absorbed by the chemical system. If this energy transfer between system and surroundings occurs rapidly, flames or the emission of light might be evident. A chemistry student should always be on the lookout for such observations when in lab. They are not just trivial happenstance and should always be noted when observed.
Because the product chemicals are different than the reactant chemicals, changes are commonly observed in the system. The changes that are observed serve as evidence that a chemical reaction has taken or is taking place. A color change is often observed. Bubbles can be observed being formed in the system or an odor that was not previously present might be detected. A solid might be formed at the bottom of a solution (known as a precipitate). On occasion there is a noticeable change in temperature of the surroundings as energy is released from or absorbed by the chemical system. If this energy transfer between system and surroundings occurs rapidly, flames or the emission of light might be evident. A chemistry student should always be on the lookout for such observations when in lab. They are not just trivial happenstance and should always be noted when observed.
Chemical Reactions at a Particle Level
Chemical reactions are everywhere. Methane gas burning on your kitchen stovetop. Food digesting in your stomach. A wood log burning in your fireplace. An antacid tablet reacting to ease some digestive problems. Octane gasoline fueling a car’s movement along a roadway. Lactose reacting as milk sours in the refrigerator. Propane gas reacting to heat up your outdoor grill. The bubbling of an Alka-Seltzer tablet in water. The iron of a car body slowly rusting over time. A candle quietly burning on the fireplace mantle. These are all chemical reactions. They all involve reactants changing into products. How can we understand the details of what is happening?
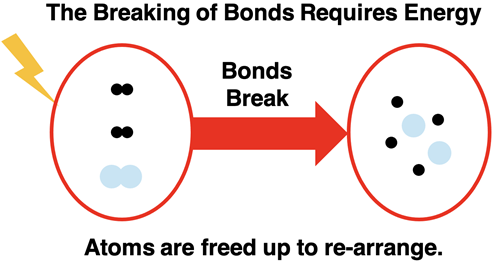
 Let’s start with a simple example – hydrogen gas reacting with oxygen gas to form water. The reactants are the starting materials; that’s hydrogen and oxygen. Both are diatomic gases (remember Professor H2O2N2Cl2Br2I2F2) with two atoms in a molecule. For the reaction to occur the bonds that hold atoms of hydrogen and atoms of oxygen together must break. Energy is required by the system of two chemicals to break apart the bonds. The impact of a high-speed collision between two molecules moving randomly about the container provides enough energy to break apart the bonds. The process is often facilitated by providing a spark. However it starts, energy is required.
Let’s start with a simple example – hydrogen gas reacting with oxygen gas to form water. The reactants are the starting materials; that’s hydrogen and oxygen. Both are diatomic gases (remember Professor H2O2N2Cl2Br2I2F2) with two atoms in a molecule. For the reaction to occur the bonds that hold atoms of hydrogen and atoms of oxygen together must break. Energy is required by the system of two chemicals to break apart the bonds. The impact of a high-speed collision between two molecules moving randomly about the container provides enough energy to break apart the bonds. The process is often facilitated by providing a spark. However it starts, energy is required.
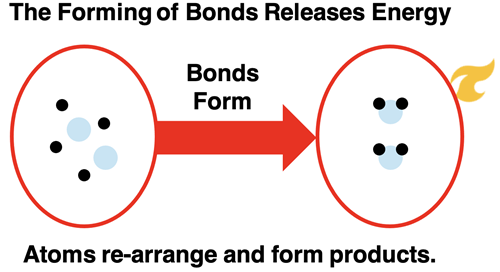
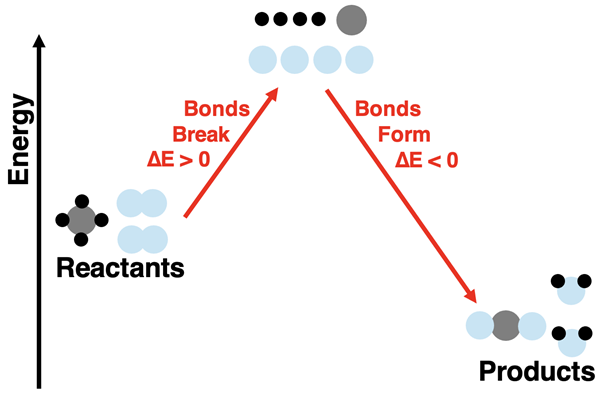
Breaking the bonds that hold atoms together in the hydrogen and oxygen molecules frees the atoms up to undergo a rearrangement. The most common rearrangement pathway is for two hydrogen and one oxygen atom to form a new molecule. The product molecule is water (H2O). The energy of the product is less than that of the free atoms. The bond formation step lowers the energy of the system.
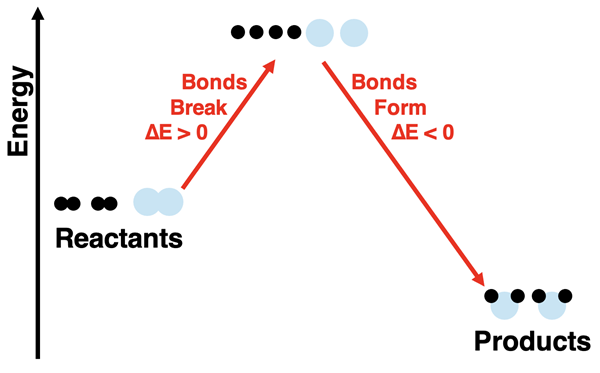
There are two energy changes involved in this reaction process. There is the energy change associated with breaking bonds and the energy change associated with forming new bonds. If we refer to the chemicals (reactants and products) as the system, then the bond breaking step (∆Ebreaking bonds) results in a positive energy change in the system. The bond forming step (∆Eforming bonds) results in a negative energy change of the system. (The ∆ symbol means change.) The overall energy change for the reaction depends on how the energy change of these two steps compare. In the specific case of hydrogen and oxygen reacting to form water, the products have a lower energy than the reactants.
One final note: you might have observed that there are two molecules of hydrogen reacting for every one molecule of oxygen. This is not an arbitrary decision on our part or a mere exercise of artistic license. As we will learn on the next page of this Lesson, the ratios by which reactant particles combine is governed by a larger principle known as the law of conservation of mass.
Your Kitchen Stove
A common stove in a kitchen uses natural gas as a fuel. The gas is burned to heat or cook your food. The reaction involves methane gas (CH4) provided by the stove reacting with oxygen gas (O2) provided by the surrounding air. The reaction is initiated by a spark; a knob is turned that causes an igniter to create sparks. Let’s use our model of reactions to analyze the reaction of methane gas with oxygen gas.
The model suggests that the bonds in the reactant molecules are first broken. Once broken, the atoms are free to rearrange and form new bonds, resulting in the products. The products are carbon dioxide (CO2) and water (H2O).
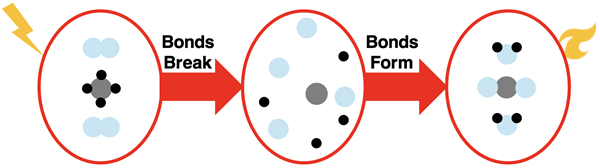
Similar to the previous reaction, this reaction also results in a net loss of energy by the system. While energy is required to break the bonds, the formation of products releases more energy than what was required. The loss of energy by the system is what makes the reaction useful for heating or cooking food on a stovetop.
Explaining the Evidence
Reactions involve the breaking of bonds, the rearrangement of atoms, and the formation of new bonds. Reactant chemicals are turned into product chemicals. Because new product chemicals are formed, there would be observable changes. As discussed above, reactions leave a trace of evidence. The color of the products could be different than the reactants. If so, then a color change would be observed. If the products have a characteristic odor, your nose might detect evidence that a reaction occurred. One or more of the products could be a gas and bubbles might be observed. Or one of the products could be a solid and you would observe a precipitate forming at the bottom of the container. Finally, the energy of reactants differs from the energy of the products. The result is that a temperature change would be detected in the surroundings. The container holding the chemicals might be observed to warm up or cool down. By placing your hand near the container without touching it, you can often feel the temperature change.
Before You Leave
- Download our Study Card on What is a Chemical Reaction? Save it to a safe location and use it as a review tool.
- The Check Your Understanding section below includes questions with answers and explanations. It provides a great chance to self-assess your understanding.
Check Your Understanding
Use the following questions to assess your understanding. Tap the Check Answer buttons when ready.
1. Which of the following is not a visual signal that a reaction has taken place?
- A color change is observed.
- There is a temperature change.
- A substance changes its state.
- Bubbles are observed.
- A solid precipitate is observed to form.
2. Which statement best describes what happens during a chemical change?
- Atoms are destroyed; new ones are formed.
- Molecules are destroyed; new ones are formed.
- Atoms are rearranged and regrouped.
- Molecules are rearranged and regrouped.
3. The chemicals present before a reaction takes place are called _____ and those that are present after the reaction are called _____.
- antecedents, postcedents
- pre-cursors, aftermath
- atoms, molecules
- reactants, products
4. Identify the following statements as being
True or
False. If False, correct the statement.
- Pulling apart atoms requires energy.
- Putting atoms back together to form molecules uses up energy.
- If the bond-breaking steps require more energy than the energy released by bond-forming steps, then the overall reaction has a positive energy change.
- The ∆Energy for the breaking of bonds in atoms is a positive value.
- Because bond-breaking requires energy and bond forming releases energy, the two effects balance each other and the overall energy change is zero.
- If the product chemicals are at a lower energy than the reactant chemicals, then more energy is required to break the bonds in reactants than is released by forming bonds in products.
- The ∆E for a reaction is negative if it takes relatively lower energy to pull atoms apart than the energy released when putting atoms together to form products.