Hold down the T key for 3 seconds to activate the audio accessibility mode, at which point you can click the K key to pause and resume audio. Useful for the Check Your Understanding and See Answers.
Lesson 1: Energy and Heat
Part c: Chemical Reactions and Energy
Part a: Energy
Part b: Heat and Temperature
Part c: Chemical Reactions and Energy
Part d: Calorimetry
Part e: Energy and Changes of State
Changes in Chemical Potential Energy
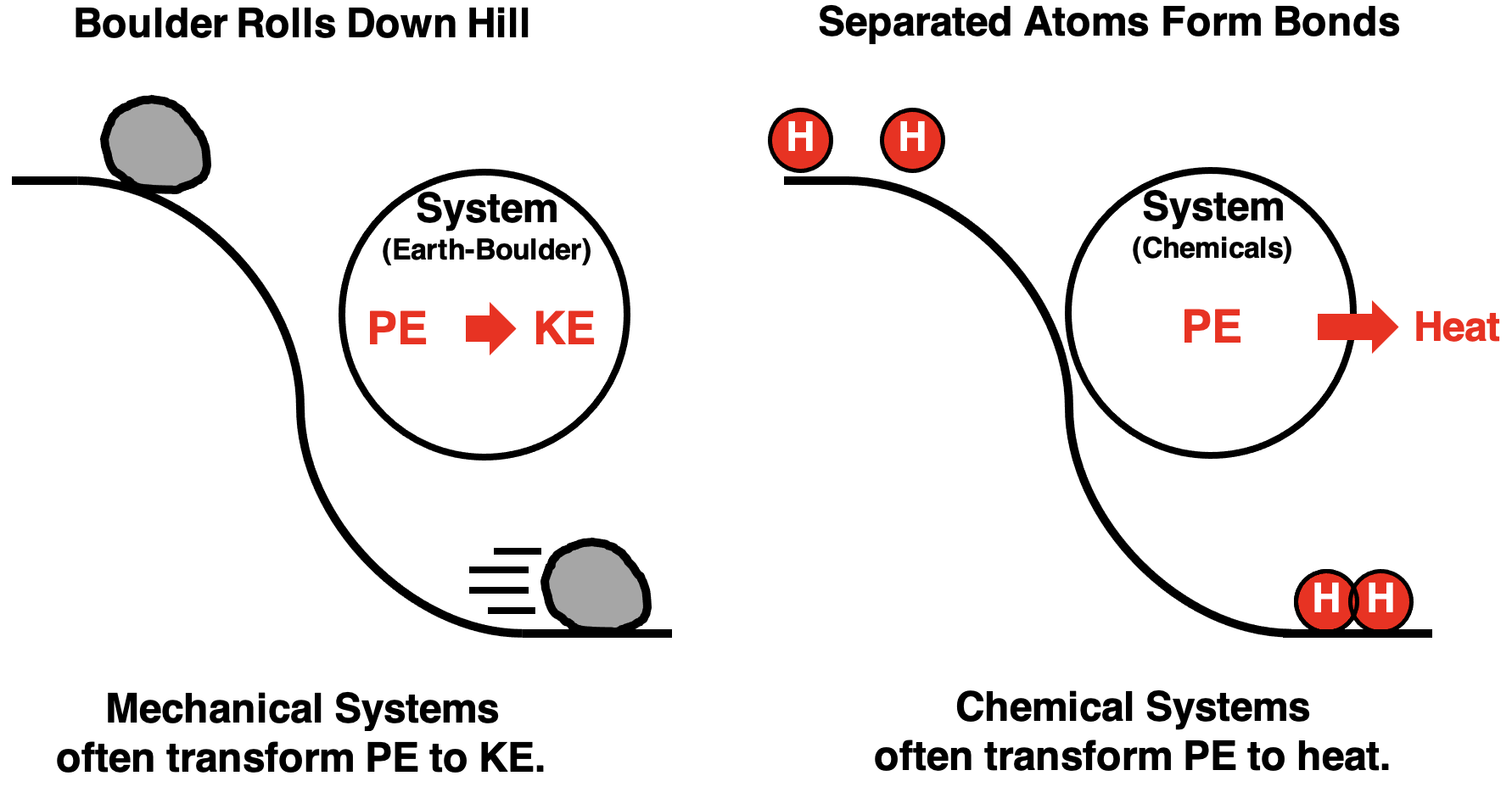
 Potential energy was discussed earlier in Lesson 1. It is the energy stored in a system due to the interactions of objects within the system. It is often thought of as the energy of position.
Potential energy was discussed earlier in Lesson 1. It is the energy stored in a system due to the interactions of objects within the system. It is often thought of as the energy of position.
Imagine a massive boulder in an unstable position on the top edge of a hill. It has gravitational potential energy relative to the bottom of the hill due to its gravitational interaction with Earth. If the boulder is given a gentle push, it will roll down the hill to a more stable position. As it rolls downward it will lose its potential energy. The law of energy conservation would suggest that the loss of potential energy would be accompanied by a gain in some other form of energy. So, as the boulder loses potential energy, it gains kinetic energy. This commonly occurs for mechanical systems.
In the case of chemical systems involving atoms, the chemical potential energy is the result of the electrical interactions between the protons and electrons of different atoms. The “top of the hill” in a chemical system is separated atoms. As those separated atoms approach to form bonds, there is a lowering of the potential energy. The law of energy conservation would suggest that the loss of potential energy would be accompanied by a release of energy to the surroundings. The system loses energy; the surroundings gain energy. So as bonds form, heat is produced. This commonly occurs for chemical systems.
 Bond Breaking and Bond Forming
Bond Breaking and Bond Forming
In Chapter 6 of this Chemistry Tutorial, our discussion of covalent bonds began with what is generally referred to as a potential energy well diagram. The animated form is shown at the right. The animation shows a system of two atoms. The atoms approach each other from a relatively large separation distance (A) to relatively small separation distance (D). This approach of atoms is placed upon a graph of the potential energy of the system versus the distance between the atoms.
The location of closest approach (D) is not the most stable positioning of the atoms; proton-proton repulsions are so strong that this position is very unstable. The location of maximum distance (A) is also not the most stable positioning of the atoms. The protons and electrons are too far apart for their attractions to have a stabilizing effect. At location B, there is a perfect balance between the stabilizing proton- electron attractions and the destabilizing proton-proton repulsions. The bottom of the well - Location B - represents the most stable positioning of atoms. The separation distance associated with Location B represents the lowest chemical potential energy. This explains why bond formation is an energy-releasing event.
electron attractions and the destabilizing proton-proton repulsions. The bottom of the well - Location B - represents the most stable positioning of atoms. The separation distance associated with Location B represents the lowest chemical potential energy. This explains why bond formation is an energy-releasing event.
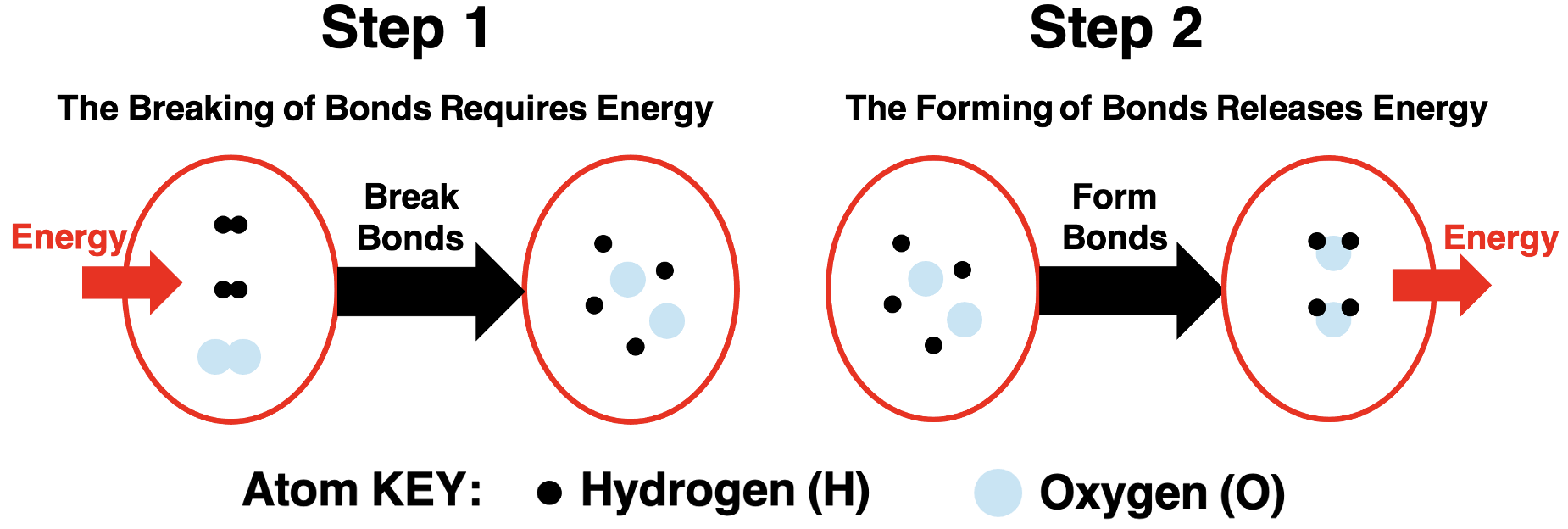
Bond formation involves a lowering of the chemical potential energy of the system. What does bond-breaking involve? Bond breaking is the opposite of bond forming. We would expect the opposite energy effects. If bond formation lowers the potential energy, then bond breaking would increase the chemical potential energy. In order for a bond to be broken, energy must be absorbed from the surroundings. Bond breaking is an energy-absorbing event.
There are three conclusions to be drawn from the potential energy well diagram:
- Separated atoms are designated as zero chemical potential energy.
- Forming bonds lowers the system’s PE and releases energy to the surroundings.
- Breaking bonds increases the system’s PE and absorbs energy from the surroundings.
(Tap to learn more about PE.)
Chemical Reactions Revisited
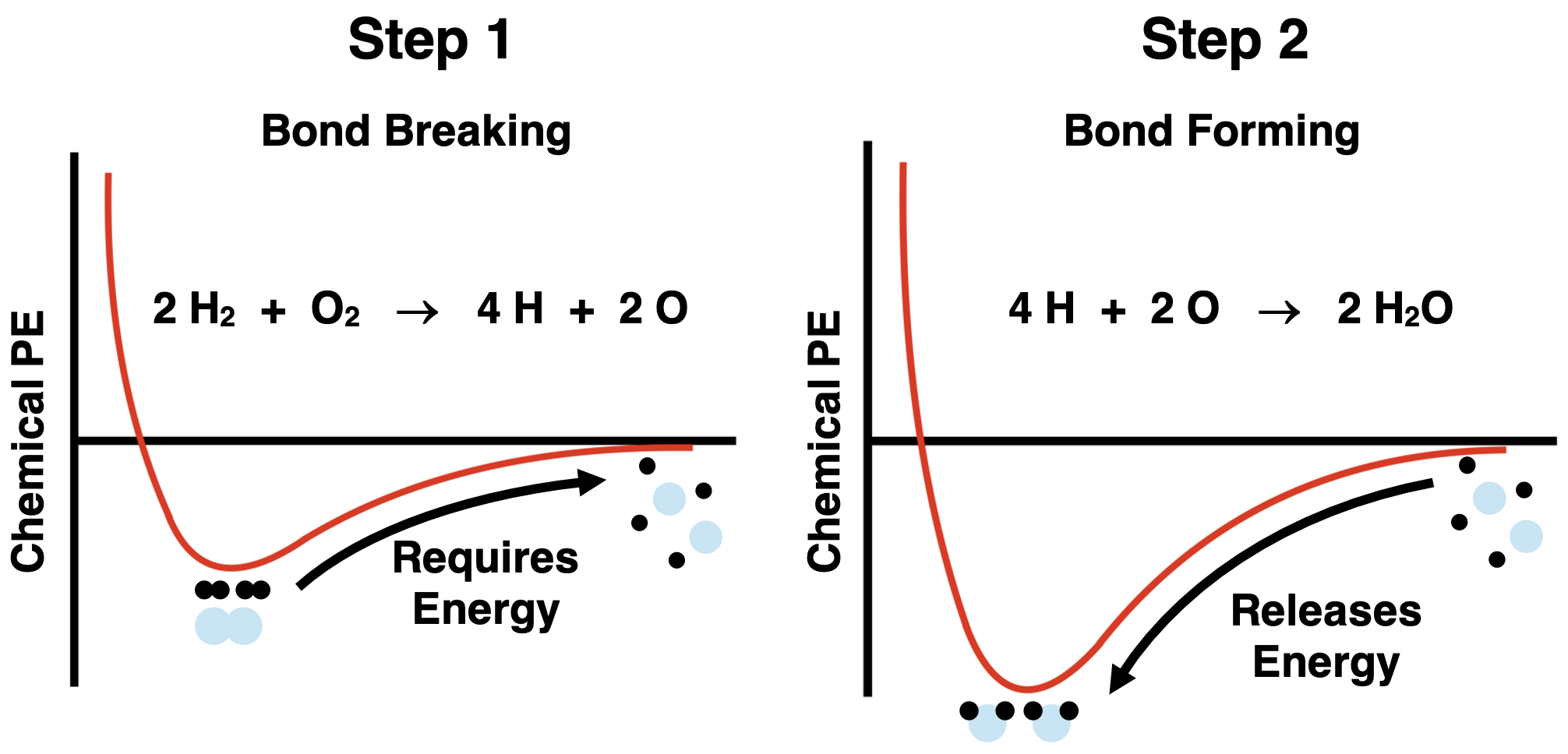
In the Chemical Reactions chapter of our Chemistry Tutorial, we learned that chemical reactions involve the breaking of bonds among reactant molecules, the rearrangement of atoms, and the formation of new bonds to form reaction products. We think of these bond-breaking and bond-forming events as occuring within the system. Let’s consider a simple reaction like the synthesis of water vapor (H2O) from its elements - hydrogen (H2) and oxygen (O2). The balanced chemical reaction is
2 H2(g) + O2(g) → 2 H2O(g)
We can think of the reaction as occurring in two steps - a bond breaking step and a bond forming step.
The bond breaking step can be thought of as starting at the bottom of the potential energy well and progressing to the point of separated atoms (below left). This is an uphill climb on the potential energy curve and would require energy from the surroundings. Once atoms are separated, they rearrange and new bonds are formed to produce products (below right). This is a downhill climb on the potential energy curve and would release energy to the surroundings.
With two steps involved, there are two energy changes:
- ∆EBond Breaking: The energy required when breaking bonds (a positive value).
- ∆EBond Forming: The energy released when forming bonds (a negative value).
The overall change in energy of the reaction is the sum of the energy changes for these two steps. If more energy is required when breaking bonds than is released when forming bonds, then the overall energy change will be positive. There is a net energy gain of the system. On the other hand, if less energy is required when breaking bonds than is released when forming bonds, then the overall energy change will be negative. There is a net energy loss of the system. In the case of the water synthesis reaction above, the overall reaction has a negative potential energy change. Like all combustion reactions, energy is released to the surroundings in the form of heat.
Energy Level Diagrams
 The analysis above utilizes two potential energy well diagrams - one for each step of the reaction. A simpler diagram is an energy level diagram. It is very similar to the well diagram except that it puts the reaction pathway from reactants to products on the same graph.
The analysis above utilizes two potential energy well diagrams - one for each step of the reaction. A simpler diagram is an energy level diagram. It is very similar to the well diagram except that it puts the reaction pathway from reactants to products on the same graph.
The diagram at the right is an energy level diagram for the synthesis of water. An energy level diagram shows the relative energy of reactants and of products along the vertical axis. The vertical axis is labeled as energy or potential energy. The horizontal axis displays a label like Reaction Progress, Reaction Pathway, or Reaction Coordinate. It represents the progress or extent of the reaction from reactants to products. There is always an initial upward curve; bonds must first be broken and that requires energy. Then there is a downward curve since energy is released during the bond-forming step. The energy of products can be lower or higher than the energy of reactants, depending on the particular reaction. The overall change in energy is the difference between the reactant’s energy level and the product’s energy level.
Endothermic and Exothermic Processes
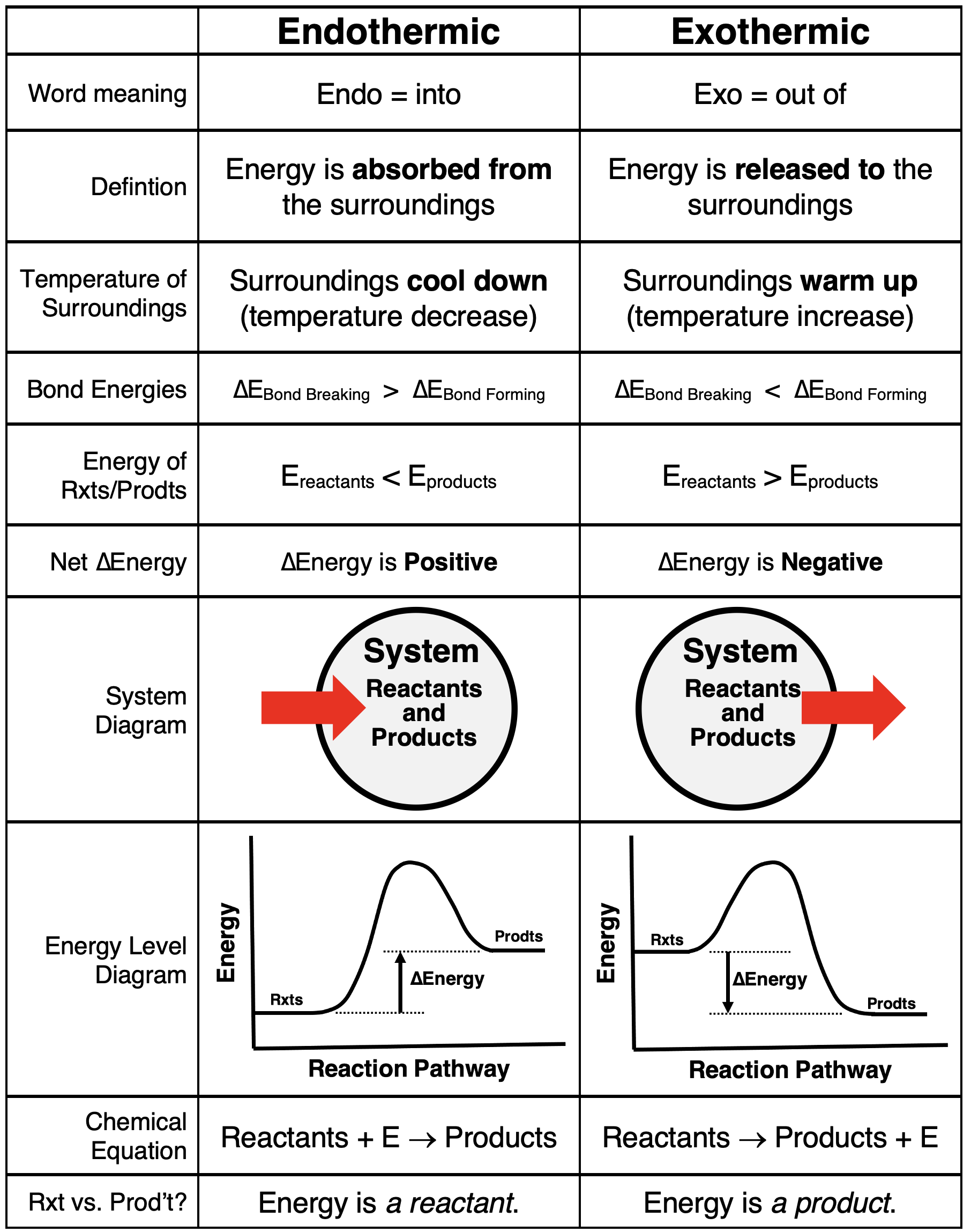
 Chemical reactions are classified as being either endothermic or exothermic. An endothermic reaction is a reaction which absorbs energy from the surroundings. This lowers the temperature of the surroundings. Energy enters the system in order for the reaction to take place. There is an overall increase in chemical potential energy within the system because the energy required for the bond breaking step is greater than the energy released during the bond forming step. Because the energy of products is greater than the energy of reactants, the system experiences an overall positive energy change.
Chemical reactions are classified as being either endothermic or exothermic. An endothermic reaction is a reaction which absorbs energy from the surroundings. This lowers the temperature of the surroundings. Energy enters the system in order for the reaction to take place. There is an overall increase in chemical potential energy within the system because the energy required for the bond breaking step is greater than the energy released during the bond forming step. Because the energy of products is greater than the energy of reactants, the system experiences an overall positive energy change.
 An exothermic reaction is just the opposite. Energy is released from the system to the surroundings causing the temperature of the surroundings to increase. There is an overall decrease in chemical potential energy, corresponding to an energy change value that is negative. The chemical potential energy of products is lower than that of reactants because the energy released during the bond forming step is greater than the energy required during the bond breaking step.
An exothermic reaction is just the opposite. Energy is released from the system to the surroundings causing the temperature of the surroundings to increase. There is an overall decrease in chemical potential energy, corresponding to an energy change value that is negative. The chemical potential energy of products is lower than that of reactants because the energy released during the bond forming step is greater than the energy required during the bond breaking step.
The table below provides a summary of energy changs for endothermic and exothermic reactions.
Thus far our discussion has been conceptual in nature. There have been no numbers. On the next page of this lesson, we will learn how to determine the numbers. A method referred to as calorimetry allow us to determine the quantity of heat that is absorbed from or released to the surroundings. But before you fast forward to that page, take some time to make sure that you comprehend these important ideas by doing one or more the suggestions mentioned in the Before You Leave section.
Before You Leave
Check Your Understanding
Use the following questions to assess your understanding. Tap the Check Answer buttons when ready.
1. Which two statements are true of a chemical system when two isolated atoms come together to form a bond? Pick two answers.
- The chemical potential energy of the system increases.
- The chemical potential energy of the system decreases.
- Energy is gained by the system from the surroundings
- Energy is released by the system to the surroundings
2. Which two statements are true of a chemical system when the bond between atoms is broken? Pick two answers.
- The chemical potential energy of the system increases.
- The chemical potential energy of the system decreases.
- Energy is gained by the system from the surroundings
- Energy is released by the system to the surroundings
3. Which statements are true about the
bottom of the well in a potential energy well diagram for two atoms? Select all that apply.
- It is the separation distance at which the most energy is stored in the system.
- It is the most stable separation distance for the two atoms.
- There are no proton-proton repulsions at this separation distance.
- It is the lowest potential energy value for the system.
- The atoms would naturally and explosively spring apart from this separation distance.
- The deeper that the well is, the more energy that would be required to break the bond.
4. The change in chemical potential energy is _______ for a bond breaking process and _______ for a bond-forming process.
- positive, positive
- negative, negative
- positive, negative
- negative, positive
5. Consider the following statements. Identify each as being characteristic of either an endothermic or an exothermic reaction.
- The temperature of the surroundings decrease.
- The potential energy of the reactants is greater than the potential energy of the products.
- More energy is required to break the bonds in the reactants than the energy released when forming bonds for the products.
- On an energy level diagram, the energy of reactants is lower than the energy of the products.
- Heat is released to the surroundings from the system.
- There is an overall negative energy change.
- The system diagram shows an arrow (representing energy or heat flow) from the surroundings to the system.
- When writing the balanced chemical equation, energy is shown as a product in the reaction.