Atomic Models - Questions
The Atomic Models Concept Builder is comprised of four different Matching Pairs activities. In order to complete any of the activities, learners must successfully associate four pairs of ideas. In the first activity -
Scientists and Models - learners associate the name of a scientist with the name of the atomic model that they developed. In the second activity -
Discoveries and Models - learners associate a specific discovery or experiment with a description of a model that the finding led to. In the third activity -
Electrons and Models - learners associate the name of a model with a description of what the model uniquely proposes about the electron. And in the fourth activity -
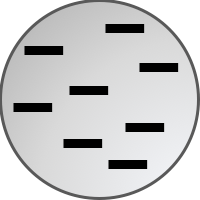
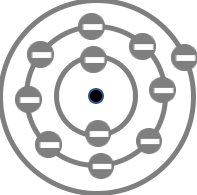
Diagrams and Models - learners associate a pictoral representation of a model with the name of the model. The Concept Builder scrambles the arrangement of ideas within the Matching Pairs grid. Otherwise, all students will have the same set of terms.
Teachers are encouraged to view the questions in order to judge which activities are most appropriate for their classes.
The Physics Classroom grants teachers and other users the right to print these questions for private use. Users are also granted the right to copy the text and modify it for their own use. However, this document should not be uploaded to other servers for distribution to and/or display by others. The Physics Classroom website should remain the only website or server from which the document is distributed or displayed. We also provide a PDF that teachers can use under the same conditions. We have included a link to the PDF near the bottom of this page.
Atomic Models
This activity presents learners with 8 different statements that must be matched by meaning. Learners tap on the statements to select them and then tap on the Check Match button. The order of the statements is randomized. A mis-matched pair restarts the
game and re-randomizes the order of the statements. The statements are ...
Activity 1: Scientists and Models
Version 1
Negative charges embeded in a uniform sea of positive charge.
Electrons orbit at only certain distances that have a discrete energy level.
Bohr's Quantum Model
Positive charge and atomic mass is concentrated in a central location.
Electrons are in orbitals, regions of space with known energy.
Rutherford's Nuclear Model
Schrodinger's Quantum Mechanical Model
J.J. Thomson's Plum Pudding Model
Activity 2: Discoveries and Models
Electrons are located in regions of space that have a precisely known energy.
A magnet deflects a cathode ray beam in a spedific direction.
When bombarded by alpha particles, a gold foil deflects the particles from their path.
Atom contains a densely packed nucleus with + charge.
Electrons in atoms have discrete, quantized energy values.
One cannot simultaneously know both the location and momentum of an object.
The line spectra of hydrogen are explained by electrons changing orbits.
Atoms contain negatively charged particles (i.e., electrons).
Activity 3: Electrons and Models
Electrons are located in regions of space known as orbitals.
Neils Bohr Model
There are negatively-charged electrons embedded in the atom.
Dalton Model
Electrons are orbitting a nucleus like planets orbit the sun.
Electron? What's an electron?
J.J. Thomson Model
Quantum Mechanical Model
Activity 4: Representation and Scientist/Model
Schrodinger's Quantum Mechanical Model
J.J. Thomson Plum Pudding Model
Dalton Atomic Model
Neils Bohr's Quantum Model