Cell Voltage - Questions
The Cell Voltage Concept Builder is comprised of 24 questions organized into 12 Question Groups and spread across three difficulty levels. Each question requires the same task - using a Reduction Potential Table to analyze a galvanic cell and determine the cell voltage. There is little variation in difficulty between the difficulty levels. They should be thought of as additional examples to practice with.
Here is the breakdown of the difficulty levels:
- Apprentice Difficulty Level: Question Groups 1-4 ... Students are given contents of each half-cell and must use a table of standard reduction potentials to determine the reduction and oxidation half equations and their potential and the overal cell voltage.
- Master Difficulty Level: Question Groups 5-8 ... Students are given contents of each half-cell and must use a table of standard reduction potentials to determine the reduction and oxidation half equations and their potential and the overal cell voltage.
- Wizard Difficulty Level: Question Groups 9-12 ... Students are given contents of each half-cell and must use a table of standard reduction potentials to determine the reduction and oxidation half equations and their potential and the overal cell voltage.
The questions from each group are shown below. Teachers are encouraged to view the questions in order to judge how many difficulty levels are most appropriate for their classes. Alternatively, teachers can do the
Concept Builder to gain a feel for the student experience..
The Physics Classroom grants teachers and other users the right to print these questions for private use. Users are also granted the right to copy the text and modify it for their own use. However, this document should not be uploaded to other servers for distribution to and/or display by others. The Physics Classroom website should remain the only website or server from which the document is distributed or displayed. We also provide a PDF that teachers can use under the same conditions. We have included a link to the PDF near the bottom of this page.
Cell Voltage
Apprentice Difficulty Level
Question Group 1
Question 1
Determine the half-reactions and their potential and the overall cell voltage for this galvanic cell:
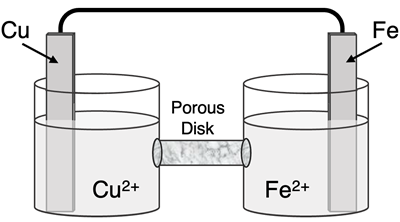
Reduction Half-Equation:
Oxidation Half-Equation:
Ered =
Eox =
Ecell =
Question 2
Determine the half-reactions and their potential and the overall cell voltage for this galvanic cell:
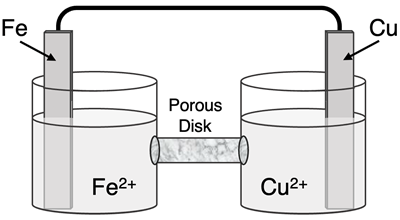
Reduction Half-Equation:
Oxidation Half-Equation:
Ered =
Eox =
Ecell =
Question Group 2
Question 3
Determine the half-reactions and their potential and the overall cell voltage for this galvanic cell:
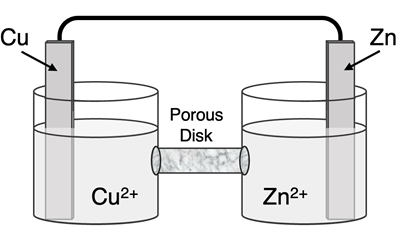
Reduction Half-Equation:
Oxidation Half-Equation:
Ered =
Eox =
Ecell =
Question 4
Determine the half-reactions and their potential and the overall cell voltage for this galvanic cell:
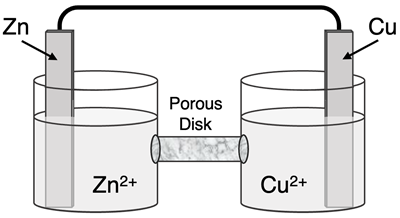
Reduction Half-Equation:
Oxidation Half-Equation:
Ered =
Eox =
Ecell =
Question Group 3
Question 5
Determine the half-reactions and their potential and the overall cell voltage for this galvanic cell:

Reduction Half-Equation:
Oxidation Half-Equation:
Ered =
Eox =
Ecell =
Question 6
Determine the half-reactions and their potential and the overall cell voltage for this galvanic cell:
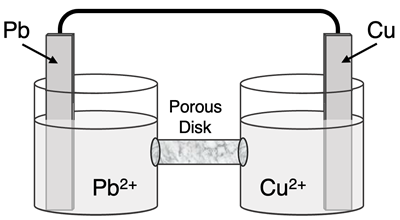
Reduction Half-Equation:
Oxidation Half-Equation:
Ered =
Eox =
Ecell =
Question Group 4
Question 7
Determine the half-reactions and their potential and the overall cell voltage for this galvanic cell:
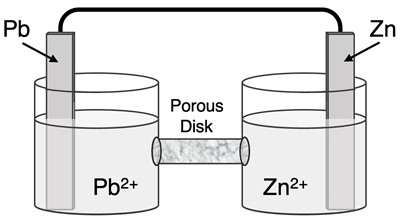
Reduction Half-Equation:
Oxidation Half-Equation:
Ered =
Eox =
Ecell =
Question 8
Determine the half-reactions and their potential and the overall cell voltage for this galvanic cell:
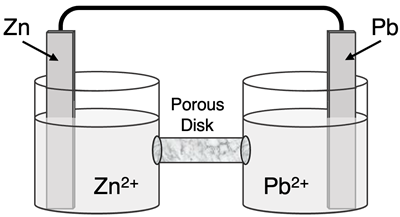
Reduction Half-Equation:
Oxidation Half-Equation:
Ered =
Eox =
Ecell =
Master Difficulty Level
Question Group 5
Question 9
Determine the half-reactions and their potential and the overall cell voltage for this galvanic cell:
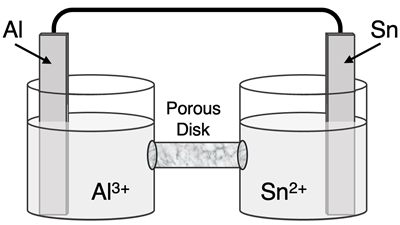
Reduction Half-Equation:
Oxidation Half-Equation:
Ered =
Eox =
Ecell =
Question 10
Determine the half-reactions and their potential and the overall cell voltage for this galvanic cell:
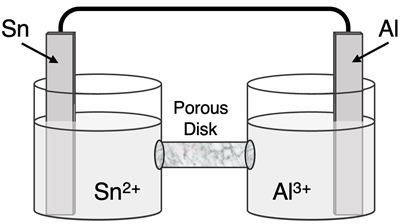
Reduction Half-Equation:
Oxidation Half-Equation:
Ered =
Eox =
Ecell =
Question Group 6
Question 11
Determine the half-reactions and their potential and the overall cell voltage for this galvanic cell:
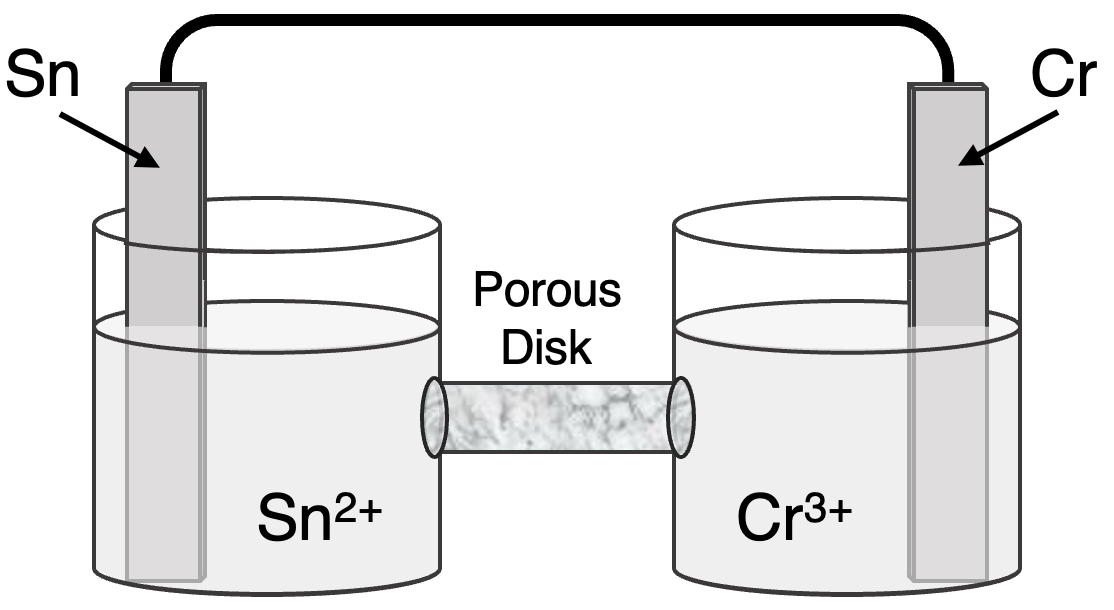
Reduction Half-Equation:
Oxidation Half-Equation:
Ered =
Eox =
Ecell =
Question 12
Determine the half-reactions and their potential and the overall cell voltage for this galvanic cell:
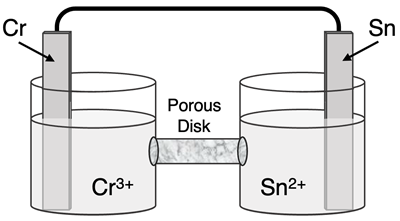
Reduction Half-Equation:
Oxidation Half-Equation:
Ered =
Eox =
Ecell =
Question Group 7
Question 13
Determine the half-reactions and their potential and the overall cell voltage for this galvanic cell:
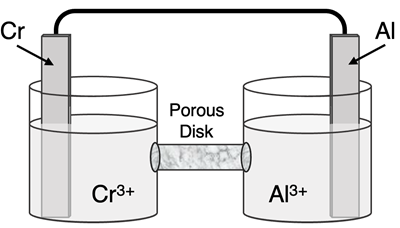
Reduction Half-Equation:
Oxidation Half-Equation:
Ered =
Eox =
Ecell =
Question 14
Determine the half-reactions and their potential and the overall cell voltage for this galvanic cell:
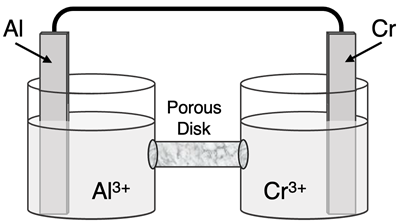
Reduction Half-Equation:
Oxidation Half-Equation:
Ered =
Eox =
Ecell =
Question Group 8
Question 15
Determine the half-reactions and their potential and the overall cell voltage for this galvanic cell:
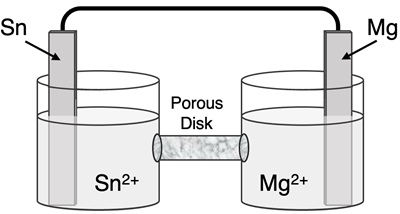
Reduction Half-Equation:
Oxidation Half-Equation:
Ered =
Eox =
Ecell =
Question 16
Determine the half-reactions and their potential and the overall cell voltage for this galvanic cell:
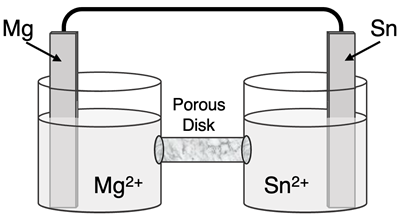
Reduction Half-Equation:
Oxidation Half-Equation:
Ered =
Eox =
Ecell =
Wizard Difficulty Level
Question Group 9
Question 17
Determine the half-reactions and their potential and the overall cell voltage for this galvanic cell:
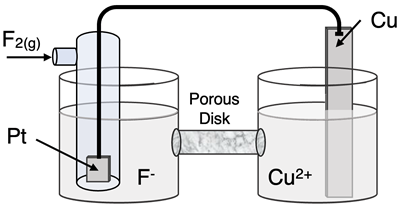
Reduction Half-Equation:
Oxidation Half-Equation:
Ered =
Eox =
Ecell =
Question 18
Determine the half-reactions and their potential and the overall cell voltage for this galvanic cell:
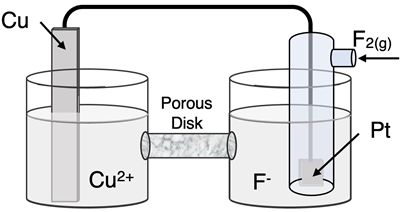
Reduction Half-Equation:
Oxidation Half-Equation:
Ered =
Eox =
Ecell =
Question Group 10
Question 19
Determine the half-reactions and their potential and the overall cell voltage for this galvanic cell:
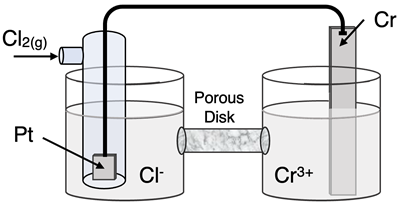
Reduction Half-Equation:
Oxidation Half-Equation:
Ered =
Eox =
Ecell =
Question 20
Determine the half-reactions and their potential and the overall cell voltage for this galvanic cell:
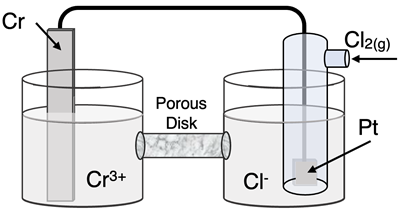
Reduction Half-Equation:
Oxidation Half-Equation:
Ered =
Eox =
Ecell =
Question Group 11
Question 21
Determine the half-reactions and their potential and the overall cell voltage for this galvanic cell:
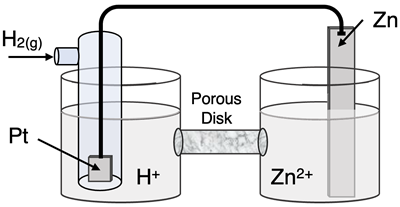
Reduction Half-Equation:
Oxidation Half-Equation:
Ered =
Eox =
Ecell =
Question 22
Determine the half-reactions and their potential and the overall cell voltage for this galvanic cell:
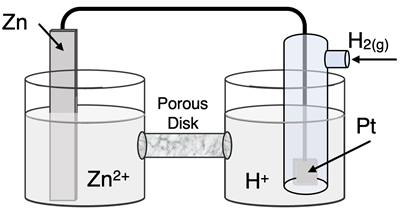
Reduction Half-Equation:
Oxidation Half-Equation:
Ered =
Eox =
Ecell =
Question Group 12
Question 23
Determine the half-reactions and their potential and the overall cell voltage for this galvanic cell:
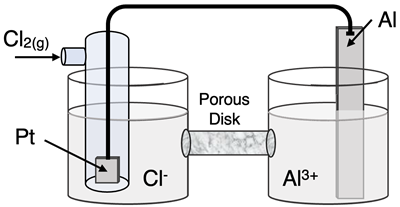
Reduction Half-Equation:
Oxidation Half-Equation:
Ered =
Eox =
Ecell =
Question 24
Determine the half-reactions and their potential and the overall cell voltage for this galvanic cell:
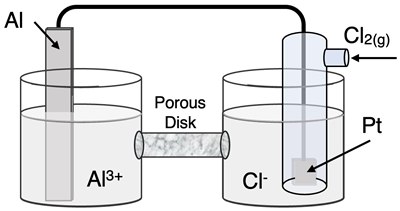
Reduction Half-Equation:
Oxidation Half-Equation:
Ered =
Eox =
Ecell =