Hold down the T key for 3 seconds to activate the audio accessibility mode, at which point you can click the K key to pause and resume audio. Useful for the Check Your Understanding and See Answers.
Lesson 3: Gas Stoichiometry
Part a: Stoichiometry Revisited
Part a: Stoichiometry Revisited
Part b:
Gas Stoichiometry at Standard Conditions
Part c:
Gas Stoichiometry at Non-Standard Conditions
The How Much? Questions of Chemical Reactions
We discussed the topic of Stoichiometry in Chapter 9. Stoichiometry will be a sub-topic of nearly every unit for the remainder of our Tutorial. Why? Because stoichiometry pertains to the quantitative nature of chemical reactions and chemical reactions is central part of what a Chemistry course is all about.
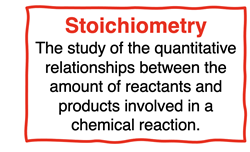
Stoichiometry is the study of the quantitative relationship between the amounts of reactants and products involved in a chemical reaction. Stoichiometry focuses on the How much? questions of chemical reactions. Those How much? questions require a student to relate the amount of reactants to the amount of products. In Chapter 9, those amounts, or How much? quantities included …
- moles of a reactant
- moles of a product
- mass of a reactant (in grams)
- mass of a product (in grams)
The task of solving a stoichiometry problem requires an understanding of
what is related to what? and
how are they related?
Mole Ratios and Molar Mass
In Chapter 9, success at stoichiometry required that a student understand and be able to use mole ratios and molar mass. To illustrate, let’s consider the reaction for the synthesis of ammonia:
N2(g) + 3 H2(g) → 2 NH3(g)
The coefficients 1, 3, and 2 in front of the formulae N
2, H
2, and NH
3 indicate how many moles of reactants and products are involved in the chemical reaction. They assist in answering all how much moles? type questions. Conversion factors can be made from these coefficients to convert between moles of reactants and moles of product or between moles of the two reactants.
The
molar mass of a substance is the mass in grams of 1 mole of that substance. Molar mass values can be found using a periodic table and then used to convert between grams of a substance and moles of that same substance.
Coefficients (mole ratios), molar mass values, and the factor label method are the tools used to answer the how much? questions of stoichiometry. Their use was demonstrated through numerous examples in
Chapter 9. If you find yourself a bit rusty on the topic, we encourage you to make a return visit to
Chapter 9 and tool up. In this chapter, we will apply the stoichiometry tools that we have learned to reactions that involve one or more gaseous reactant or product.
The New How Much? Quantities
Gas stoichiometry problems will introduce new How much? quantities to our existing four quantities. Those quantities are
volume, pressure, and temperature. We learned in Lesson 2 that the
Ideal Gas Equation relates the number of moles of a gas to the volume, pressure, and temperature of the gas. We also learned in Lesson 2 that
Avogadro’s Law predicts that two different gases held at the same pressure and temperature will have the same ratio of volume to moles. So, our list of How much? quantities expands to include the volume, pressure, and temperature of reactants and products.
- moles of a reactant
- moles of a product
- mass of a reactant (in grams)
- mass of a product (in grams)
- volume, pressure, and temperature of reactant
- volume, pressure, and temperature of product
Avogadro’s Law and Gas Stoichiometry
In the ammonia synthesis reaction …
N2(g) + 3 H2(g) → 2 NH3(g)
… all reactants and products are in the gas state. The coefficients indicate the mole ratios with which they involve themselves in the reaction.
Avogadro’s Law suggests that if present at the same pressure and temperature, the coefficients also indicate the
volume ratios. For instance, 1 L of N
2 gas would react with 3 L of H
2 gas to produce 2 L of NH
3 gas. Knowing the volume of a reactant would allow us to predict the volume of the other reactants. Coefficients take on expanded meaning for gaseous reactants and products present at the same temperature and pressure.
Molar Volume and Gas Stoichiometry
The concept of a
molar volume was introduced in Lesson 2. The molar volume is the volume per 1 mole of gas. We learned that at
Standard Temperature (273.15 K) and Pressure (1 atm), all gases have
a molar volume of 22.4 L/mol. While molar mass relates the mass of a substance to the moles of a substance, molar volume relates the volume of a gaseous substance to the moles of the same gaseous substance. And at
STP conditions, the numerical relationship between volume and moles is 22.4 L = 1 mole. Molar volume can be used to form conversion factors for converting between moles and liters for a gaseous substance at STP.
The PIVNERT Equation and Gas Stoichiometry
The Ideal Gas Law was introduced in Lesson 2. The
Ideal Gas Law equation, P•V = n•R•T, allows one to relate the number of moles (
n) of a gaseous substance to the volume (
V) at a specified pressure (
P) and temperature (
T). Unlike the molar volume figure of 22.4 L/mole, the so-called
PIVNERT equation can be used at any temperature and pressure conditions to relate the number of moles of a gas to its volume. The equation can be rearranged to determine the moles from the volume or the volume from the moles.
Two Types of Problems
We will see two types of gas stoichiometry problems in the remaining pages of Lesson 2.
The first type of gas stoichiometry problem will involve reactants and products at Standard Temperature and Pressure (STP). At STP, the molar volume is 22.4 L/mol. This value can be used to relate volume to moles for gaseous substances. We will see numerous examples in Lesson 3b.
The second type of problem will involve reactants and products at non-STP conditions. At such non-STP conditions, the
PIVENERT equation must be used to relate volume to moles. For both STP and non-STP conditions, we can relate the volumes of reactants and products to one another using the coefficients provided that the substances are gases and present at the same pressure and temperature.
Before You Leave
- Problem Set GL15 in our Calculator Pad section provides six quick volume-to-volume conversions for gaseous reactants and products. It’s great practice! Visit Problem Set GL15: Gas Stoichiometry 1.
- The Check Your Understanding section below include questions with answers and explanations. It provides a great chance to self-assess your understanding.
Check Your Understanding
Use the following questions to assess your understanding. Tap the Check Answer buttons when ready.
1. Can the molar volume value of 22.4 L/mol be used in reactions involving solids and liquids at STP?
2. Can the
PIVNERT equation be used in reactions involving solids and liquids?
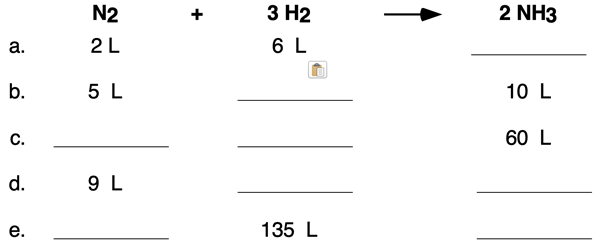
3. Complete the following table by indicating the missing volumes for the reaction
N2(g) + 3 H2(g) → 2 NH3(g)
Assume reactants and products are at the same temperature and pressure.