Hold down the T key for 3 seconds to activate the audio accessibility mode, at which point you can click the K key to pause and resume audio. Useful for the Check Your Understanding and See Answers.
Lesson 3: Molecular Shape
Part c: Molecular Polarity
Part 3a:
Valence Shell Electron Pair Repulsion Theory (VSEPR)
Part 3b:
Advanced VSEPR
Part 3c: Molecular Polarity
Part 3d:
Hybridized Orbital Theory
 Polar vs. Nonpolar Bonds
Polar vs. Nonpolar Bonds
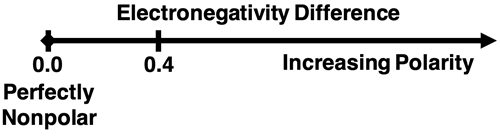
A covalent bond can have a range of polarities. On one end of the spectrum is the purely nonpolar bond in which electrons are shared equally among bonded atoms. On the other end of the spectrum is the very polar covalent bond in which electrons are shared unequally. The difference in electronegativity of the two atoms determines where on the spectrum that a bond lies. A general rule of thumb is that two atoms with a difference in electronegativity of 0.4 or less is a nonpolar covalent bond. The electrons are shared so equally among the two atoms that there is minimal to no partial charges on the atoms. For electronegativity differences greater than 0.4, the bond can be considered a polar covalent bond. Increases in the difference in electronegativity values correspond to a greater amount of polarity. (Use our Table of Electronegativities to make judgements about the polarity of a bond.)
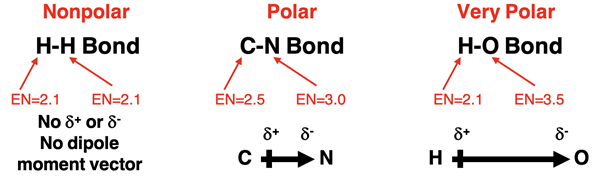
 Dipole Moment Vectors
Dipole Moment Vectors
As discussed in Lesson 2, a polar bond exhibits a partial positive charge (δ+) and partial negative charge (δ-). This dipole (separation of positive and negative charge) is often represented by a dipole moment vector. The term vector indicates a quantity that has a direction associated with it in addition to size or numerical value. By definition, the direction of a dipole moment vector is from the positive partial charge towards the negative partial charge. Put another way, the dipole moment vector for a bond is directed from the least electronegative atom towards the most electronegative atom. The size or numerical value is proportional to the difference in electronegativities for the two bonded atoms.
Three bonds are shown below. The H-H bond is nonpolar. Electrons are shared equally and there are no partial positive or negative charges. Without a dipole, there is no dipole moment vector. The C-N bond is polar. The more electronegative N atom has the partial negative charge (δ-) and the C atom has the partial positive charge (δ+). The dipole moment vector is directed from the carbon to the nitrogen. The H-O bond is also polar. The more electronegative O atom has the partial negative charge (δ-) and the H atom has the partial positive charge (δ+). The dipole moment vector is directed from the hydrogen to the oxygen. The dipolar moment for the H-O bond has a larger size than the dipole moment for the C-N bond. The size of the vector arrow in the diagram is larger for the H-O bond.
 Molecular Polarity
Molecular Polarity
Consider a molecule with several bonds. If the bonds are polar, then there is more than one dipole moment vector. Each polar bond has its own dipole moment vector. It is important to distinguish between the polarity of a bond and the polarity of a molecule. A molecule can have polar bonds but still be a nonpolar molecule. How can this be?
Because dipole moments are vectors with a direction, they are subject to the rules of vectors. Oppositely directed vectors of the same size can cancel each other. This doesn’t need to be overly complicated (yet). As an example, force is a vector that causes objects to accelerate from their position. Yet two oppositely directed forces of the same size will cancel their effect upon the object such that it remains as is. Dipole moment vectors are like force vectors. They can cancel each other out so that there is no overall net effect. Molecules can be nonpolar if the dipole moment vectors of the individual polar bonds cancel each other’s effect. This explains why a molecule can have polar bonds yet be a nonpolar molecule.
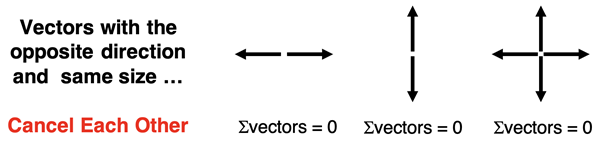
Cancellation of Dipole Moment Vectors
Oppositely directed dipoles of the same size cancel each other. But those are not the only conditions that lead to vector cancellation. The difficulty with Lesson 3c will be to get a grasp of when two or more vectors cancel and when they don’t. We will discuss various molecular geometries that lead to vector cancellation and those that don’t. The full topic is often discussed in a math class or a physics class. Diving into the details of vectors is usually more convincing but takes several class days. We will be briefer.
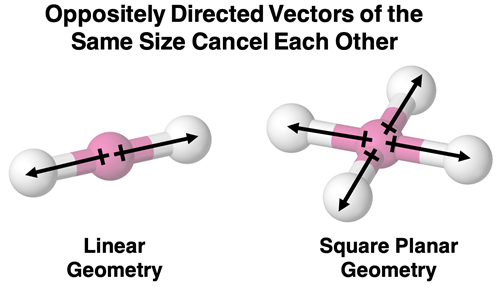
Let’s begin with two of the more obvious cancellation conditions: linear and square planar molecular geometries. If all dipoles are of the same size, cancellations occurs.
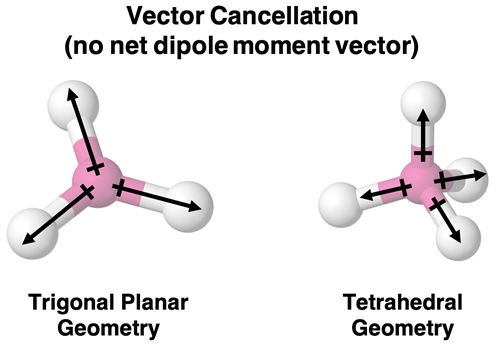
Two less obvious cancellation conditions include trigonal planar and tetrahedral geometries. If there is an identical dipole moment vector for each bond, then the three vectors (trigonal planar) and the four vectors (tetrahedral) will cancel each other’s effect. There would be no net dipole moment vector and an overall nonpolar molecule.
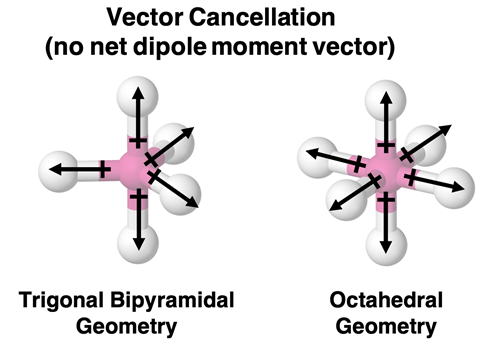
The following two geometries also lead to vector cancellation if all dipoles are of equal size. Trigonal bipyramidal can be pictured as a blend of the trigonal planar condition and the linear condition. Octahedral can be pictured as a blend of the square planar condition and the linear condition.
 Now let’s consider some conditions in which two identically sized dipole moment vectors do not cancel each other. A bent molecular geometry is our most obvious example. Because the two vectors are not directed exactly opposite each other, they will not cancel.
Now let’s consider some conditions in which two identically sized dipole moment vectors do not cancel each other. A bent molecular geometry is our most obvious example. Because the two vectors are not directed exactly opposite each other, they will not cancel.
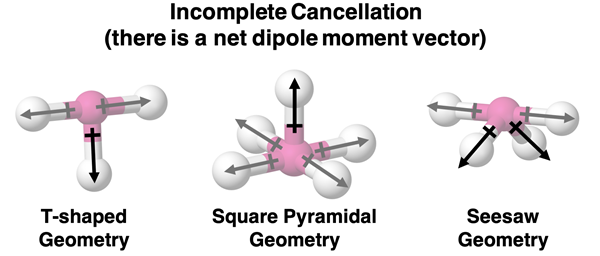
T-shaped, square pyramidal, and seesaw geometries do not result in cancellation of vectors. In each of these geometries, there are two or even four of the multiple dipole moment vectors that do cancel (greyed out on diagram). However, there are one or two vectors that are not being cancelled. If just one of the dipole moment vectors does not cancel, then there will be a net dipole moment and the molecule will have overall polarity.
 Trigonal pyramidal is a final situation in which there is no cancellation of the dipole moment vectors. This situation should not be confused with trigonal planar. Trigonal planar exists in a single plane whereas trigonal pyramidal extends above (or below) the plane. This extension into the third dimension explains why the pyramidal form leads to incomplete vector cancellation while the planar form does result in cancellation.
Trigonal pyramidal is a final situation in which there is no cancellation of the dipole moment vectors. This situation should not be confused with trigonal planar. Trigonal planar exists in a single plane whereas trigonal pyramidal extends above (or below) the plane. This extension into the third dimension explains why the pyramidal form leads to incomplete vector cancellation while the planar form does result in cancellation.
Method for Determining Molecular Polarity
A common task in an introductory Chemistry course is to identify the polarity of a molecule if given its name or chemical formula. The following method will prove useful in determining the polarity of a molecule.
- Use electronegativity values to determine if there are any polar bonds in the molecule. If there is exactly one polar bond, then the molecule is polar. If there are two or more polar bonds, then continue with steps 2 - 4.
- Draw the Lewis electron dot diagram.
- Determine the molecular geometry.
- Use the molecular geometry to picture the spatial orientation of the bonds. Based on the information in the Cancellation of Dipole Moment Vectors (above), determine if the dipole moment vectors cancel each other out. If they do cancel, then the molecule is nonpolar. If the dipole vectors do not cancel, then the molecule is polar.
Caution will have to be given in situations that contain two or more dipole moment vectors but the size of the vectors are not the same. This is common in molecules with three or more elements. Our discussion assumed equal-sized dipole moment vectors. Non-equal sized dipole moment vectors do not cancel when they are directed in opposite directions.
The following flow chart will assist with your decision-making.
Before You Leave
- Download our Study Card on Molecular Polarity. Save it to a safe location and use it as a review tool.
- Practice! Try our Concept Builder titled Molecular Polarity. It’s awesome practice.
- The Check Your Understanding section below include questions with answers and explanations. It provides a great chance to self-assess your understanding.
Check Your Understanding
Use the following questions to assess your understanding. Tap the Check Answer buttons when ready.
1. Use our Table of Electronegativities and the rule of thumb to identify the following bonds as being polar or nonpolar.
- N-F
- B-H
- P-Cl
- B-Cl
- H-P
2. Use our
Table of Electronegativities to rank the following bonds in order of their polarity from least to greatest.
N-F, C-H, H-F, P-O
3. Draw the dipole moment vectors on the following electron dot diagrams. Use our
Table of Electronegativities.

4. Aaron Agin is insisting that PCl
5 shown in Diagram A above is polar. His argument is that the molecule has polar bonds and there is no way that they will cancel since they are not in opposite diretions. Talk to Aaron and explain his error.
5. Aaron Agin is again insisting that BCl
3 shown in Diagram C above is polar. Aaron argues that two of the three dipole moments for BCl
3 will cancel and the remaining one will be uncancelled. Talk to Aaron and explain his error.
6. Use our
Table of Electronegativities and the proposed method to determine if the following molecules are polar. Give reasons for each conclusion that you make.
- NH3
- SF4
- CH4
- OCl2
- CCl4
- CH2Cl2
- XeF4
- BH3
- BCl3