Hold down the T key for 3 seconds to activate the audio accessibility mode, at which point you can click the K key to pause and resume audio. Useful for the Check Your Understanding and See Answers.
Lesson 2: Covalent Bonding
Part b: Lewis Electron Dot Structures
Part 2a:
Covalent Bonds
Part 2b: Lewis Electron Dot Structures
Part 2c:
Double and Triple Bonds
Part 2d:
Octet Breakers
Part 2e:
Formal Charge Considerations
What is a Lewis Electron Dot Diagram?
In 1916, American chemist Gilbert Newton Lewis proposed the concept of covalent bonding as the sharing of an electron pair between two atoms. Lewis proposed that atoms shared pairs of valence electrons with one another in an effort to achieve an electron configuration identical to a noble gas configuration. Each atom attracts the electron pair. This attraction is the nature of the covalent bond that holds atoms together. The electrons in the shared electron pair count towards the octet of both atoms.
Lewis introduced the idea of an electron dot diagrams to represent the bonding pairs of electrons shared between atoms and the unshared pairs of electrons owned by atoms. In electron dot diagrams, electrons are represented by dots. The diagrams group dots (electrons) together in pairs and position them around the atoms. An atom is represented by its elemental symbol. Electrons positioned between two different atoms are the bonding pairs. They are often replaced by a line to represent a bond between atoms. Each line represents a single bond. Lewis electron dot diagrams are sometimes referred to as Lewis structures, Lewis diagrams, electron dot structures, and electron dot diagrams.
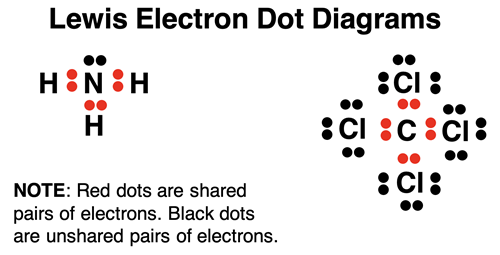
Lewis Electron Dot Diagrams for Isolated Atoms
Lewis electron dot diagrams only account for the location of valence shell electrons. These are the s and p orbital electrons found in the outermost electron shell of an atom. To demonstrate how they are constructed for an isolated atom, let’s consider the nonmetal elements in period 2. The table below shows the orbital box diagram and the electron configuration for these five nonmetals. The number of valence electrons is very obvious from these two representations. This number is used to determine the number of dots in the electron dot diagrams. The first four dots are entered as unpaired dots (see row 1). These unpaired dots would be electrons that would be shared with another atom to form a covalent bond. The fifth dot gets paired up (see row 2). It is not important which side of the symbol the first electron pair is drawn on – top, bottom, left, or right. The same can be said of the second, and third electron pairs. Atoms share their unpaired electrons with other atoms to form covalent bonds. The number of bonds that we would expect the atom to form is equal to the number of unpaired electrons in the dot diagram. Observe that neon (Ne) already has a full octet. It would not form covalent bonds with other atoms. Neon is a noble gas. As the name implies, the nobility of the early 20th century tended to not mix with other elements of society.
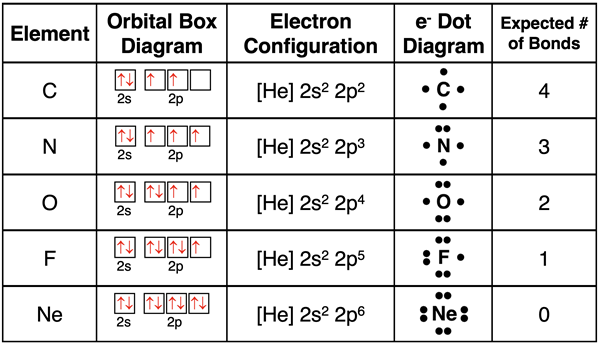
The Covalent Bond
 Electron dot diagrams are generally more useful when drawn for molecules as opposed to atoms. Just like for atoms, the electron pairs (pairs of dots) in the diagram represent valence shell electrons. They are always paired (unless the atom has an odd number of electrons … a rare and radical situation). The pairs can be …
Electron dot diagrams are generally more useful when drawn for molecules as opposed to atoms. Just like for atoms, the electron pairs (pairs of dots) in the diagram represent valence shell electrons. They are always paired (unless the atom has an odd number of electrons … a rare and radical situation). The pairs can be …
- Shared pairs, bonding pairs, bonds … all synonymous terms, or
- Unshared pairs, non-bonding pairs, lone pairs … also synonymous terms.
Shared pairs are usually replaced by lines. A line represents
a single bond. Consider a line to be two electrons shared among atoms. (In
Lesson 2c, we will learn about
double bonds and triple bonds.)
Counting the Number of Valence Shell Electrons
An important skill is to be able to count the number of valence electrons for a molecule. The task involves adding up all the
valence shell electrons for the individual atoms. A subscript in the formula becomes a multiplier of the number of valence electrons for that particular element. Here are three examples. (Excuse Xenon for acting less than noble.)
 Central Atoms and Terminal Atoms
Central Atoms and Terminal Atoms
A Lewis electron dot structure shows how the various atoms are structured. Most of the examples we use will include simple structures with a
central atom. The other atoms in the molecule will attach (i.e., bond) to the central atom. These are referred to as
terminal atoms. There is some skill to identifying the central atom and the terminal atoms.
With few exceptions, the central atom is usually the lone element in the formula. It is the C of CCl
4 and the P of PCl
3 and the N of NH
3 and the C of CO
2. The central atom is most often the atom of the first element listed in the formula. It is the C of CH
3Cl. Finally, the central atom is often the least electronegative element. These are just guidelines that cover 95% of the cases. The fact is that the central atom could follow neither of these guidelines. An educated guess is still a guess.
Perhaps the most important point to note is that there is a central atom and the other atoms bond to it. For the examples done in this unit, you can count on the fact that the atoms of the molecule won’t be forming the shape of a hexagon, pentagon, rectangle, triangle, or Sponge Bob SquarePants.
Drawing Electron Dot Diagrams for Molecules
It is best to following a systematic procedure for drawing electron dot diagrams.
Winging it usually leads to dissatisfying results. We have found the following four step procedure to be helpful. Avoid shortening it to three steps.
- Count the number of valence shell electrons in the molecule.
- Form a skeleton structure for the molecule. Use bonds (lines) to connect terminal atoms to the central atom.
- Using pairs of dots (electron pairs), add lone pairs to give all atoms an octet of electrons.
- Count the number of electrons in the diagram (bonds count as two electrons). Then compare to the Step 1 count of the number of valence electrons.
- If the number of electrons from step 4 equals the number of valence shell electrons from step 1, you’re done!
- If the number of electrons from step 4 is greater than the number of valence shell electrons from step 1, then you will need a double or triple bond. That’s the topic of Lesson 2c.
- If the number of electrons from step 4 is less than the number of valence shell electrons from step 1, then this is a molecule that breaks the octet rule. That’s the topic of Lesson 2d.
Let’s put the rules to practice in an effort to construct a Lewis electron dot diagram for nitrogen trifluoride (NF
3).
Step 1: Count the number of valence electrons (ves) in NF
3.
N: 5 ves
F: (7 ves) * 3 = 21 ves
Total: 5 + 21 = 26 ves
Step 2: Form a skeleton structure for the molecule.
 Step 3
Step 3: Add lone pairs to give all atoms an octet of electrons.
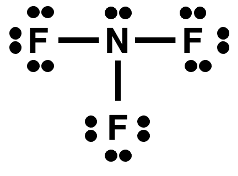 Step 4
Step 4: Count the electrons in the diagram. Compare to the number of valence electrons.
# of e
-s in diagram: 26
# of ves from Step 1: 26
They are equal. So, you’re done.
More Examples for Practice
Use a sheet of paper (or comparable medium) and our proposed procedure to draw Lewis electron dot diagrams for the following molecules. When you are done, tap the Check Answer button to check your answer. The complete solution will be shown.
Practice #1: CCl4
Practice #2: H2O
Practice #3: NBr3
Practice #4: SCl2
Practice #5: CH2Cl2
The Importance of Step 4
It is essential that you do step 4, including the comparison part of it. Step 3 is just an act of making sure every element satisfies the octet rule without regard to the number of valence electrons. There is nothing that prevents you from using more or less than the number of valence electrons determined in step 1 … except for Step 4. In the above practice examples, molecules were chosen such that the number of electrons from step 4 was always equal to the number of valence electrons from step 1. But there are plenty of instances in which this is not the case. And that’s why you must do step 4. Consider this question:
Draw the Lewis electron dot structure for CO2.
After we conduct the first three steps of the process, we end up with the dot diagram shown below in Step 3:
In Step 4, we conduct a comparison of the number of electrons in the diagram to the number of valence electrons from step 1. There are 20 electrons in the diagram. Step 1 indicated that the number of valence electrons is 16. We have used four more electrons than allowed. That means that we are not done and must make adjustments. The adjustment is to remove electrons and create multiple bonds between atoms. We are saving this discussion for Lesson 3c.
Lewis Electron Dot Diagrams for Polyatomic Ions

We discussed
polyatomic ions in Chapter 3. Polyatomic ions are a collection of two or more atoms that are held together as a bundle by covalent bonds. The number or electrons in the bundle is not equal to the number of protons. Thus, it is an ion. If the ion has a negative charge, then there are more electrons than protons. If the ion has a positive charge, then there are less electrons than protons.
Because the atoms of a
polyatomic ion are covalently bonded, it is common to construct electron dot diagrams to show how the electrons are arranged about the atoms in the ion. The same rules that are used to construct dot diagrams for molecules are used to construct dot diagrams for these
polyatomic ions. There is one slight twist that has to do with counting the number of valence electrons. We recommend that you count the number of electrons for the “neutral form” of the ion and then make adjustments for the ion charge. The adjustments are to add valence electrons for an ion with a negative charge and to subtract valence electrons for an ion with a positive charge. Examples are shown below:
Some Guidelines
Drawing electron dot diagrams can be very difficult. It requires some discipline, lots of practice, and some know-how (like a lot of things in life). The following guidelines will assist in the drawing of these diagrams.
- Always count the total number of valence electrons and use exactly the same number of dots when constructing the Lewis diagrams. Include lone pairs (non-bonding electrons), shared pairs (bonds), and multiple bonds around each atom in an effort to satisfy the duet and octet rules. We will discuss double and triple bonds in Lesson 2c.
- The octet rule (duet rule for H) is your go to rule for arranging electron pairs around atoms. But there are plenty of exceptions to the rule. Don’t be surprised when you have to make exceptions. We will help you learn how to make exceptional decisions in Lesson 2d. Until then, let the octet rule be a true rule.
- Hydrogen always satisfies its duet rule by forming a single bond to another atom.
- Second row elements C, N, O, and F will always satisfy the octet rule. There are no exceptions.
- Second row elements Be and B will usually have less than an octet of electrons. After all, if you bring only two or three electrons to the party, then it’s not realistic to expect to go home with eight.
- Third row and heavier elements will often exceed the octet rule, using d orbitals to house extra valence electrons. When drawing Lewis electron dot diagrams, satisfy the octet rule for all atoms; if electrons remain, place them on the central atom (which is often the larger atom). We will discuss situations in which the octet rule is broken in Lesson 2d.
Before You Leave
- Download our Study Card on Electron Dot Diagrams. Save it to a safe location and use it as a review tool.
- The Check Your Understanding section below include questions with answers and explanations. It provides a great chance to self-assess your understanding.
Check Your Understanding
Use the following questions to assess your understanding. Tap the Check Answer buttons when ready.
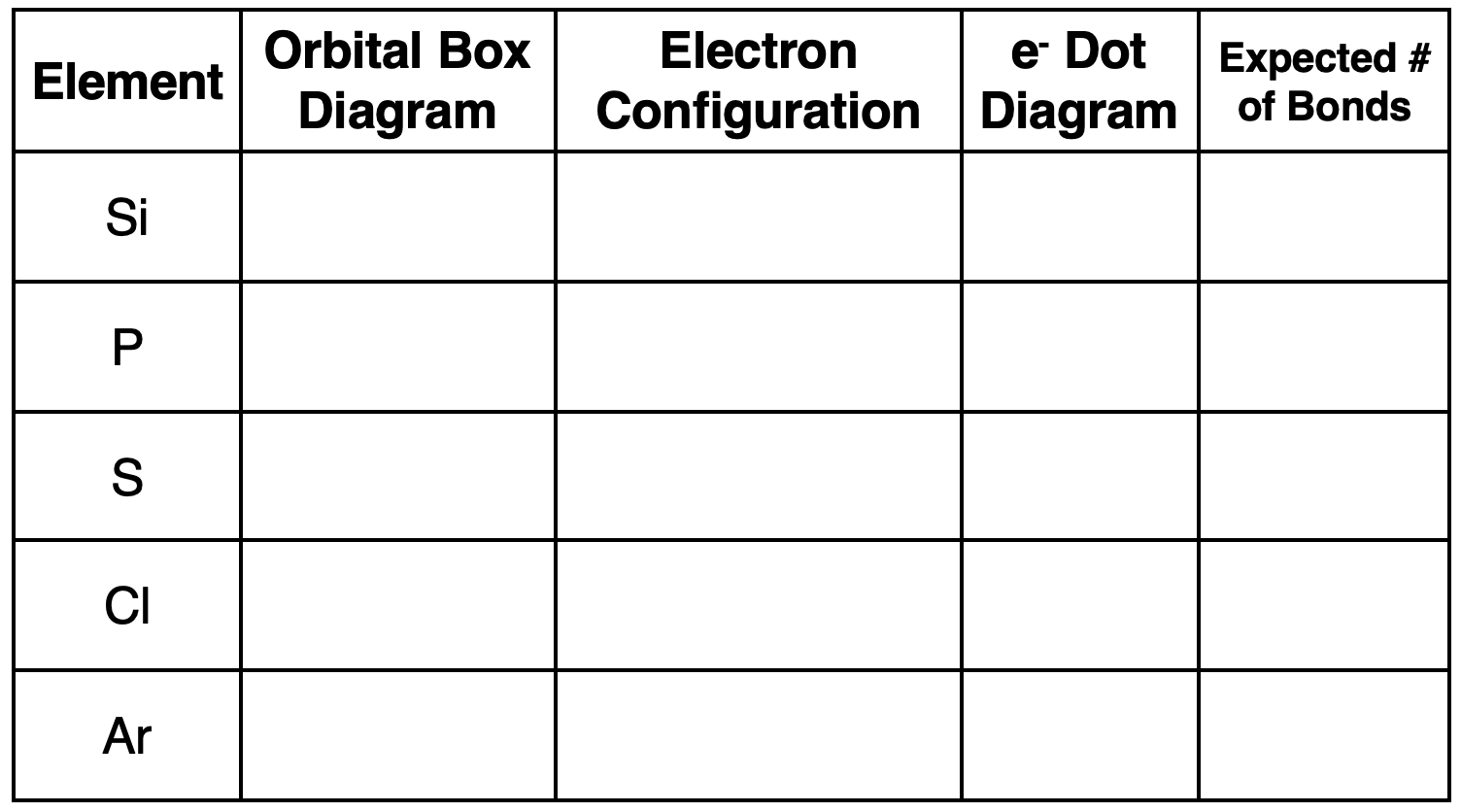
1. Complete the following table for Period 3 elements.
2. Explain how you can tell the difference between a bonding pair of electrons and a nonbonding pair of electrons.
3.
TRUE or
FALSE:
When an atom covalently bonds, it shares all of its valence electrons with the atom it is bonding with.

4. The electron dot structure for nitrogen trifluoride is shown at the right. Determine the number of …
- … shared pairs on the central N atom.
- … unshared pairs on the central N atom.
- … shared pairs on one of the terminal F atoms.
- … unshared pairs on one of the terminal F atoms.
5. Count the number of valence shell electrons on …
- CH4
- O2
- N2
- SO2
- PCl3
- PCl5
- O3
- I3- ion
- NO3- ion
- C2H3O2- ion
6. Construct the electron dot diagram for the OCl2 molecule.
7. Construct the electron dot diagram for the PCl
3 molecule.