Hold down the T key for 3 seconds to activate the audio accessibility mode, at which point you can click the K key to pause and resume audio. Useful for the Check Your Understanding and See Answers.
Lesson 1: Energy and Heat
Part b: Heat and Temperature
Part a: Energy
Part b: Heat and Temperature
Part c: Chemical Reactions and Energy
Part d: Calorimetry
Part e: Energy and Changes of State
 What is Temperature?
What is Temperature?
The question of what is temperature? seems like an easy one to answer … until you try to answer it. Answering that temperature is whatever the thermometer reads is a very accurate answer but only begs the question what is it that the thermometer measures?
We learned in Chapter 10 of our Chemistry Tutorial that the Kelvin temperature of a sample of gas is directly proportional to the average kinetic energy of its particles. The particles in a sample of gas are all moving about the container, each with its own speed. There is a range of speeds and kinetic energies possessed by its particles. The average of all these kinetic energies is directly proportional to the Kelvin temperature. The plot below represents the distribution of speeds in a sample of gas at three different temperatures. You will notice how increasing the temperature widens the distribution of speeds and increases the average speed (which is roughly but not exactly the peak of the curve).
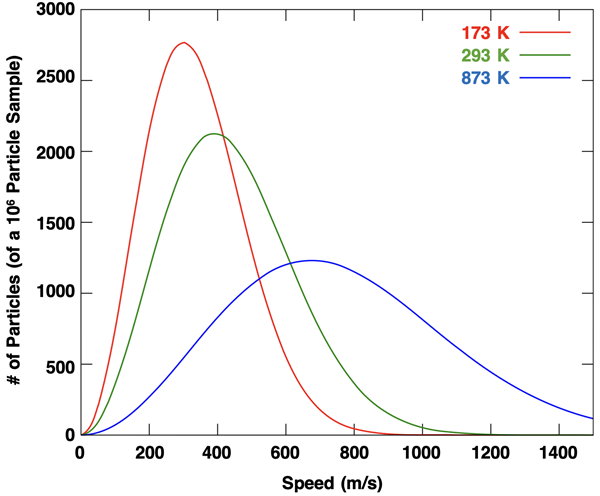
 Temperature is a measure of how energetic the particles of a sample of matter are. A high temperature gas sample is a gas sample with highly energetic particles – particles moving at relatively higher speeds (on average). And a lower temperature gas sample consists of less energetic particles moving, on average, at lower speeds. In effect, a thermometer is like a speedometer. The value that it reads provides a measure of the speed of movement of the particles. Whether it be a gas sample with particles moving about the container or a solid sample with particles vibrating about a fixed position, the temperature provides a measure of how energetic the particles are.
Temperature is a measure of how energetic the particles of a sample of matter are. A high temperature gas sample is a gas sample with highly energetic particles – particles moving at relatively higher speeds (on average). And a lower temperature gas sample consists of less energetic particles moving, on average, at lower speeds. In effect, a thermometer is like a speedometer. The value that it reads provides a measure of the speed of movement of the particles. Whether it be a gas sample with particles moving about the container or a solid sample with particles vibrating about a fixed position, the temperature provides a measure of how energetic the particles are.

Heat Transfer
 Not everything is always the same temperature. The outdoor temperature is often different than the indoor temperature. Your body temperature is different than the room temperature. The pot on the stove has a different temperature than the air in the kitchen. The ice cube from the freezer has a different temperature than the water pouring from the tap. What happens when two objects of different temperatures are placed next to each other?
Not everything is always the same temperature. The outdoor temperature is often different than the indoor temperature. Your body temperature is different than the room temperature. The pot on the stove has a different temperature than the air in the kitchen. The ice cube from the freezer has a different temperature than the water pouring from the tap. What happens when two objects of different temperatures are placed next to each other?
Suppose a mug full of freshly brewed coffee is placed on the kitchen table. There is now a hot object (mug of coffee) surrounded by lower temperature objects (table and air in the room). Gradually over time, there will be an energy transfer from the hot object to the cooler objects. We refer to this energy transfer between objects of different temperature as heat.
The result of the heat transfer is that the mug of coffee cools down. As it transfers energy to other objects, its particles become less energetic. The table may appear to become warm at the location of contact with the mug as heat is transferred to table. The room is likely so large that any increase in the air temperature a few feet away would not be detected. The mug and the coffee lower their temperature and their particles become less energetic. Eventually, the mug, the coffee, and the air in the room reach the same temperature and heat transfer ceases.
 We discussed system diagrams on the previous page. We can represent the heat transfer from the mug of coffee to the surroundings by a system diagram. Since our focus is on the mug of coffee, it would represent the system. The rest of the universe, and more specifically the table and air in the room, would be the surroundings. Energy is being transferred from the system to the surroundings. We would draw an arrow directed from the system to the surroundings. The arrow represents the transfer of energy across the system boundary to the surroundings as heat.
We discussed system diagrams on the previous page. We can represent the heat transfer from the mug of coffee to the surroundings by a system diagram. Since our focus is on the mug of coffee, it would represent the system. The rest of the universe, and more specifically the table and air in the room, would be the surroundings. Energy is being transferred from the system to the surroundings. We would draw an arrow directed from the system to the surroundings. The arrow represents the transfer of energy across the system boundary to the surroundings as heat.
 Thermal Equilibrium
Thermal Equilibrium
Let’s try a new thought experiment. Suppose we heat up a sample of metal to a high temperature (90°C) and then place it in a perfectly insulated cup filled with chilled water (10°C). And as long as it’s a thought experiment, let’s suppose that we can insert a thermometer into the metal and into the water to monitor the temperatures of each. Finally, let’s assume that the water is stirred such that it is of a uniform temperature throughout. What would we observe? And how can we explain it?
As in all situations, energy will transfer as heat from the hotter object to the cooler object. The hot metal will transfer energy to the surrounding water. Because the cup is perfectly insulated, there will be no transfer of energy to the room. From the law of conservation of energy, we know that the energy lost by the metal will be equal to the energy gained by the water. As energy is transferred out of the metal, its particles become less energetic and the thermometer readings will decrease. As for the water, its particles become more energetic and its thermometer readings will increase. This heat transfer will continue until finally the metal and the water have the same temperature. A sample plot of the temperature of the metal and water over the course of time is shown.
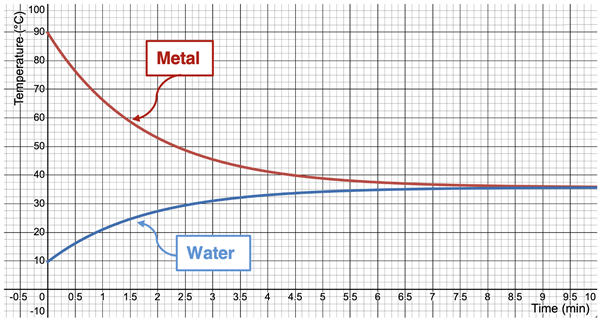
Since heat transfer only occurs when there is a difference in temperature, the transfer ceases once the water and the metal reach the same temperature. When equal temperatures are reached, the water and the metal are in a state of thermal equilibrium. Thermal equilibrium is the state in which two adjoining objects have reached the same temperature and the transfer of heat between them no longer occurs.
 In our situation, the metal started at 90°C and the water started at 10°C. The temperature at thermal equilibrium is not necessarily the midpoint temperature. The midpoint temperature is 50°C but the final temperature is 35.7°C. Does this mean the metal lost more heat than the water gained? Absolutely not! That would violate the law of energy conservation. The amount of energy lost by the metal equals the amount of energy gained by the water. But the effects of these energy changes upon temperature are not equal. The metal experiences a greater temperature change. The effect that heat gain or loss has upon temperature change depends upon the material and the amount of mass of each sample. We will learn more about this later in Lesson 1 when we discuss calorimetry.
In our situation, the metal started at 90°C and the water started at 10°C. The temperature at thermal equilibrium is not necessarily the midpoint temperature. The midpoint temperature is 50°C but the final temperature is 35.7°C. Does this mean the metal lost more heat than the water gained? Absolutely not! That would violate the law of energy conservation. The amount of energy lost by the metal equals the amount of energy gained by the water. But the effects of these energy changes upon temperature are not equal. The metal experiences a greater temperature change. The effect that heat gain or loss has upon temperature change depends upon the material and the amount of mass of each sample. We will learn more about this later in Lesson 1 when we discuss calorimetry.
Before You Leave
- Download our Study Card on Heat and Temperature. Save it to a safe location and use it as a review tool.
- Try our Science Reasoning Center Activity on Thermal Equilibrium. It’s a great follow-up to this lesson.
- The Check Your Understanding section below include questions with answers and explanations. It provides a great chance to self-assess your understanding.
Check Your Understanding
Use the following questions to assess your understanding. Tap the Check Answer buttons when ready.
1. An object is observed to be increasing its temperature. You can be certain that its particles are _____.
- getting larger
- getting more massive
- moving or vibrating with greater energy
- ready to explode
2. Heat is _______.
- the same thing as temperature
- a substance that moves around, usually from hot to cold objects
- the kinetic energy that particles have
- energy transferring from a hot object to a cold object.
3. Your friend is having troubles understanding this concept:
a thermometer is like a speedometer. Write a paragraph that explains this idea in your own words.
4. Use your newly learned chemistry understanding to explain what happens when you place an ice cube into a large glass of tap water. Identify the system and the surroundings in your explanation. Talk nerdy and use the term
thermal equilibrium in your explanation.
5. Humans maintain a body temperature of approximately 37°C. And while the temperature varies, many households maintain an inside air temperature of about 24°C. How does heat transfer apply to this situation? And why do our bodies maintain a relatively constant temperature despite being immersed in colder surroundings?