Hold down the T key for 3 seconds to activate the audio accessibility mode, at which point you can click the K key to pause and resume audio. Useful for the Check Your Understanding and See Answers.
Lesson 4: Periodic Trends
Part a: The Periodic Law Revisited
Part 4a: The Periodic Law Revisited
Part 4b:
Atomic Size
Part 4c:
Ionization Energy
Part 4d:
Electronegativity
Mendeleev’s Periodic Law
As discussed in Chapter 3, Dmitri Mendeleev published a periodic table in 1869 to provide some logical organization to the 63 known elements. His logic for the organization of elements is often termed the periodic law. In Mendeleev’s day, it was expressed as follows:
When elements are arranged in order of increasing atomic mass, one observes a periodic repetition of their chemical and physical properties.
The term atomic mass has since been replaced by the term
atomic number, referring to the number of protons in the nucleus.
Valence and Chemical Properties
Much of what was known about the chemical properties of elements in the 1860s was associated with the valence of an element. Valence pertains to the ability of an element to combine with other elements to form compounds. Chemists were able to determine the relative proportion of atoms of an element when bonding with hydrogen or oxygen or chlorine. Elements were placed in the same family if they combined with H, O, or Cl to form compounds that had the same general formula.
The diagram below shows one of Mendeleev’s original tables. We have added some colored highlights and labels.
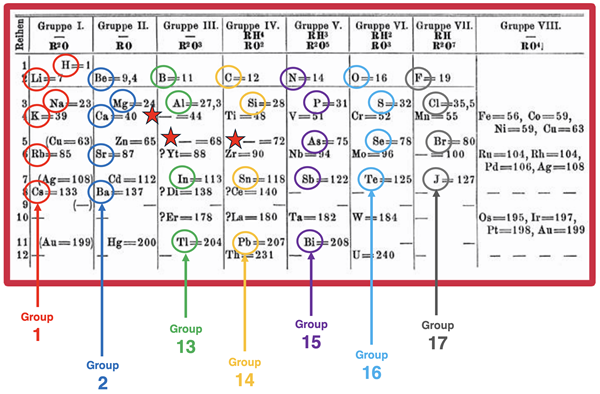
Source: Wikimedia Commons
The columns are the elemental families with similar chemical properties. Observe the generic formulae written at the top of each column. The R in a formula represents the element. The superscripts of the formulae would be correctly written as subscripts today. These subscripts indicate the relative number of atoms of R to atoms of O or H. Observe that all Gruppe I (Group 1) elements form compounds with oxygen having the form R2O (H2O, Na2O, K2O, etc.). Observe how all Gruppe V (Group 15) elements from compounds with H having the form RH3 (as in NH3, PH3, AsH3, etc.) Group V elements also combine with oxygen in a 2:5 ratio to form N2O5, P2O5, As2O5, etc.
Unlike Mendeleev, today’s chemists have the benefit of living in a world informed by the quantum mechanical model of the atom. Valence is understood to be related to the number of valence shell electrons in an atom. All Gruppe I elements and all Gruppe V elements have the same electron configuration and the same number of valence shell electrons. For metals, it is the number of valence shell electrons that affects the combining power of the element. For Mendeleev, there was no underlying cause to valence. It was simply a pattern that he was able to discern and use to organize elements into a table. Today, we know the cause.
Periodic Properties
Mendeleev’s original observation was that there were several groups of elements that shared similar chemical and physical properties. And there was a pattern to the mass of such elements. If the elements were lined up by increasing mass, it seemed that every 7th element in the line would be placed in the same group. This is what we mean by periodic – repeating or occurring at regular intervals – like every 7th element. Mendeleev was so confident in the organization of elements that he even predicted the existence of not-yet-discovered elements (X in the graphic below) with properties that would fit the emerging patterns.
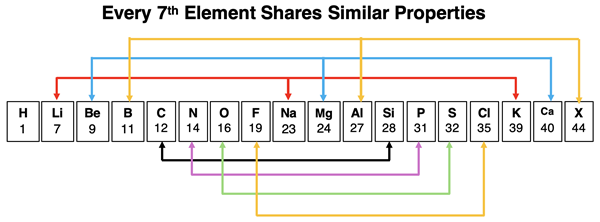
If Mendeleev’s work occurred after the discovery of the noble gases, he would have concluded that the patterns repeated themselves every 8th element. And if Mendeleev’s work occurred after the discovery of the proton, he would have argued that the properties were observed when the elements were arranged by increasing atomic number. And finally, if Mendeleev’s work had occurred after the development of quantum mechanics, atomic orbitals, and electron configurations, he would have had a clear explanation for why such trends in elemental properties existed. Mendeleev’s organization of elements is remarkable when you consider the limited knowledge base from which he made his claims.
 In Lesson 4, we will look at several properties, besides valence, that can be considered periodic properties. A periodic property is a property of an element that shows a repeating pattern of change when elements are arranged on the periodic table. One would notice the property’s value increasing or decreasing consistently when comparing its value from element to element down a column or across a row of the periodic table. The manner in which the property changes is the same down each column and across each row for the entire periodic table. We refer to the details associated with the pattern of change as a periodic trend.
In Lesson 4, we will look at several properties, besides valence, that can be considered periodic properties. A periodic property is a property of an element that shows a repeating pattern of change when elements are arranged on the periodic table. One would notice the property’s value increasing or decreasing consistently when comparing its value from element to element down a column or across a row of the periodic table. The manner in which the property changes is the same down each column and across each row for the entire periodic table. We refer to the details associated with the pattern of change as a periodic trend.
We will investigate the periodic trend for three different periodic properties:
Our attention will be on using the quantum mechanical model to explain the underlying reasons for why such trends exist.
Before You Leave
- The Check Your Understanding section below include questions with answers and explanations. It provides a great chance to self-assess your understanding.
Check Your Understanding
Use the following questions to assess your understanding. Tap the Check Answer buttons when ready.
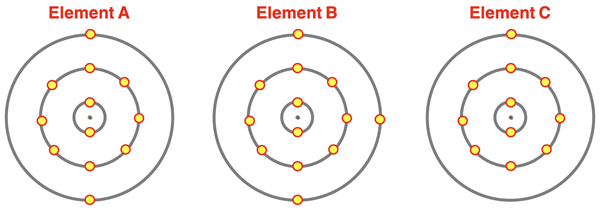
1. The diagram below shows the electron shell diagrams for unknown elements A, B, and C. Inspect the valence shell and predict the formula that the element would form with O. Use the format RxOy. where R is A, B, or C and x and y are the subscripts indicating the relative number of atoms.
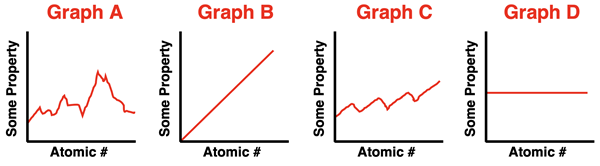
2. Suppose that there is a periodic property that is described numerically with a value. If that property value is plotted as a function of the atomic number, then what would the graph look like. Select from the four options below.