Hold down the T key for 3 seconds to activate the audio accessibility mode, at which point you can click the K key to pause and resume audio. Useful for the Check Your Understanding and See Answers.
Lesson 2: The Periodic Table Revisited
Part b: Today's Periodic Table
Part 2a: Mendeleev and the Periodic Law
Part 2b: Today's Periodic Table
Part 2c: Isotopes and Isotope Symbols
Mendeleev’s Groups
On
the previous page of Lesson 2, we discussed Dmitri Mendeleev’s efforts to organize the 63 known elements into a table based on the periodic law. If you take a casual look at this table, it may be difficult to see any correlation between it and a modern table. His table is shown below. Several of the elements have been circled to highlight the similarities between it and the modern table.
Today’s periodic table has 18 columns or groups. Mendeleev was able to identify several elements from among seven of the main groups. This includes the
32 circled elements in the table above. There were several other elements mixed in with these 32 elements that had similar chemical properties but would be regarded as part of the transition metal groups on a modern table.
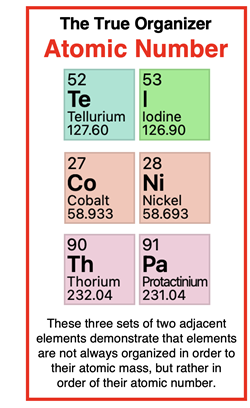
When considering the many limitations, Mendeleev’s discoveries were quite astonishing. One limitation was that the proton (and the atomic number) had not yet been discovered. Mendeleev organized the elements in order of their atomic mass. Mendeleev raised questions about the atomic mass of several elements – most notably tellurium (atomic mass=128) and iodine (atomic mass = 127). Based on properties, tellurium belonged in Group VI and the less massive iodine belonged in Group VII. Mendeleev believed the atomic mass of tellurium was measured incorrectly and changed it to 125.
A second limitation was that only 63 elements were known in Mendeleev’s day. Mendeleev left blanks in his table (see red stars on his table), believing that yet-to-be-discovered elements would someday fill in those blanks. Three examples include elements with atomic masses of 44, 68, and 72. Mendeleev predicted that these elements would soon be discovered. Scandium, gallium, and germanium were discovered within two decades of Mendeleev’s publication and had the atomic mass and chemical properties predicted by Mendeleev.
Finally, there was no theoretical basis for explaining why elements in the same group would behave similarly. It would be another half century or more before science could explain the science behind Mendeleev’s organization of elements.
 Noble Gas Discovery
Noble Gas Discovery
As mentioned, Mendeleev’s table included 63 elements. Seven of the eight main groups were included. The eighth main group that had not yet been discovered is Group 18 – the noble gases. Noble gases were discovered 2-3 decades after Mendeleev’s 1869 publication. Several of the other 118 elements were discovered in the half-century after 1869. By 1939, there were close to 90 discovered elements and the table had taken the shape of a modern table with 18 groups (columns).
Source:
https://en.wikipedia.org/wiki/File:Cumulative_diagram_of_element_discoveries.png
Mosley’s Atomic Number
In 1913, Henry Moseley developed a method of determining the number of protons in an atom of any element. Moseley bombarded samples of elements with cathode rays. The elements would emit x-rays of a measurable frequency. The frequency emitted by an element was mathematically related to the number of protons that the element possessed. Mosely discovered that every element had a unique number of protons and a unique x-ray emission spectrum. This provided an easy means of identifying an element. Moseley referred to the number of protons in an element as the
atomic number. Today’s table organizes the known elements by atomic number and not by atomic mass.
The Influence of Quantum Mechanics
In 1926, the quantum mechanical model of the atom was presented by Erwin Schrodinger. The model proposed that electrons occupy regions of space known as
orbitals. There are four types of orbitals and several
copies of each type. The maximum number of electrons that can occupy the four types of orbitals are 2, 6, 10, and 14. You might notice a pattern in the numbers.
The discovery of the quantum mechanical model of the atom provided the theoretical foundation for the organization of elements. Inspect the modern table below with the numbers 2, 6, 10, and 14 in mind. Can you see the four sections of the table that mirror the maximum electron capacity of the four orbital types? (We will discuss the many marvels of quantum mechanics in Chapter 5 of this
Chemistry Tutorial.)
The Modern Periodic Table
The modern-day table is shown above. There are 118 elements organized into 18 columns or
groups. The groups are numbered 1 through 18. There are 8 rows or
periods. The modern table includes four rows of
transition metals with 10 elements each (Groups 3-12). The table also includes two rows of 14 elements that are pulled out from the table, beginning with lanthanum and actinium. These two rows of elements are referred to as
lanthanides and
actinides. The elements are organized by increasing atomic number. Like Mendeleev’s original table, elements in the same group share similar chemical and physical properties. And in a manner similar to Mendeleev’s periodic law:
When elements are arranged in order of increasing atomic number, one observes a periodic repetition of their chemical and physical properties.
The modern table also includes atomic mass values for the elements, expressed in many cases to several decimal places. The modern table is consistent with collected data and supported by quantum mechanical theory.