Hold down the T key for 3 seconds to activate the audio accessibility mode, at which point you can click the K key to pause and resume audio. Useful for the Check Your Understanding and See Answers.
Lesson 2: Gas Laws
Part c: Pressure and Volume
Part a:
Pressure and Temperature
Part b:
Volume and Temperature
Part c: Pressure and Volume
Part d:
Volume and the Number of Moles
Part e:
The Ideal Gas Law
Part f:
Combined Gas Law
Part g:
Dalton's Law of Partial Pressure
Part h:
Graham's Law of Effusion
The Experiment
 The previous two pages of Lesson 2 presented the pressure-temperature relationship and the volume-temperature relationship. On this page, we will look at the pressure-volume relationship and, like the previous page, we will begin with an experiment.
The previous two pages of Lesson 2 presented the pressure-temperature relationship and the volume-temperature relationship. On this page, we will look at the pressure-volume relationship and, like the previous page, we will begin with an experiment.
This experiment involves an air-filled syringe with an attached pressure sensor. The syringe is calibrated to measure the volume of air inside. The volume is read from the scale and the sensor is used for pressure readings. The plunger of the syringe can be depressed and held steady with volumes ranging between 8.0 mL and 20.0 mL. Pressure readings are obtained for each volume setting. The air temperature is constant. Because the syringe is sealed at each end, the number of moles of gas is also a constant. Sample data and a graph for this experiment is shown below.
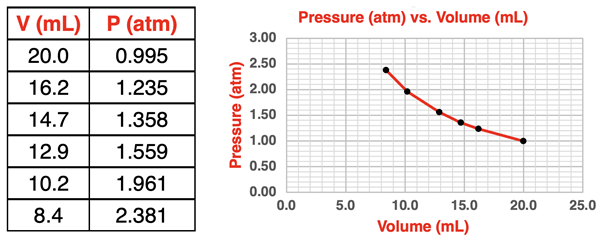
The collected data points fit a curved line. This is the pattern we might expect for two quantities that are inversely proportional to one another. One way to confirm an inversely proportional relationship is to plot Pressure vs. 1/Volume. If the plot is linear with a y-intercept of 0, then it is safe to assert that the pressure is inversely proportional to the volume. Such a plot is shown below. The red line is the best fit line for the collected data points The grey dashed line is an extension of the best fit line. The extension line passes through the origin. The y-intercept is 0.
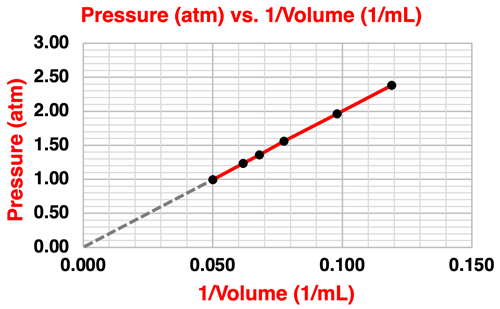
The graph confirms our hypothesis that the pressure and the volume are inversely proportional to on another. Any two quantities that are inversely proportional are described by an equation of the form x•y = k where k is a constant. For the collected data in our experiment, we might assert that …
Pressure (atm) • Volume (L) = k
The k value for our data is approximately 20.0 L•atm.
Boyle’s Law
This experiment leads us to another gas law. It is referred to as Boyle’s Law, in honor of Irish chemist Robert Boyle who is credited with its discovery. The law can be stated as follows:
For a sample of gas with a constant temperature and number of moles, the pressure of the gas is inversely proportional its volume.
This proportionality statement is often written in equation form as
P•V = k
where P is the pressure, V is the volume, and k is a proportionality constant.
 The idea of an inversely proportional relationship indicates that the factor by which the pressure is changed is the reciprocal of the factor by which the volume changes. The sample data at the right illustrate an inversely proportional relationship. A doubling of the volume causes a halving of the pressure. That is, if the volume increases by a factor of two, the pressure decreases by a factor of two. We see this pattern in Rows 1 and 2. The volume is doubled from 1.0 L to 2.0 L and the pressure is halved as a result, from 24.0 atm to 12.0 atm. Similarly, a tripling of the volume causes the pressure to be one-third of its original value. This is observed in Rows 1 and 3. The volume is tripled from 1.0 L to 3.0 L and the volume changes to 8.0 atm, which is one-third of the row 1 value of 24.0 atm. Finally, a quadrupling of the volume causes the pressure to change to one-fourth of its original value. We see this pattern in Rows 1 and 4. The volume is quadrupled from 1.0 L to 4.0 L while the pressure changes to 6.0 atm, which is one-fourth of the original pressure of 24.0 atm.
The idea of an inversely proportional relationship indicates that the factor by which the pressure is changed is the reciprocal of the factor by which the volume changes. The sample data at the right illustrate an inversely proportional relationship. A doubling of the volume causes a halving of the pressure. That is, if the volume increases by a factor of two, the pressure decreases by a factor of two. We see this pattern in Rows 1 and 2. The volume is doubled from 1.0 L to 2.0 L and the pressure is halved as a result, from 24.0 atm to 12.0 atm. Similarly, a tripling of the volume causes the pressure to be one-third of its original value. This is observed in Rows 1 and 3. The volume is tripled from 1.0 L to 3.0 L and the volume changes to 8.0 atm, which is one-third of the row 1 value of 24.0 atm. Finally, a quadrupling of the volume causes the pressure to change to one-fourth of its original value. We see this pattern in Rows 1 and 4. The volume is quadrupled from 1.0 L to 4.0 L while the pressure changes to 6.0 atm, which is one-fourth of the original pressure of 24.0 atm.
The data table also show that the product of volume and pressure is a constant value. For any given row of the table, the product is 24.0 L•atm. We could describe this data set by the equation
P•V = 24.0 L•atm.
The State Equation
There has been a steady emphasis throughout the chapter on four state variables that describe any gas sample. They are pressure, temperature, volume, and the number of moles. The values of these variables will often change. In the experiment discussed above, the volume and pressure change in an inversely proportional manner while the temperature and the number of moles were held constant. It can be said that the gas sample experienced a change of state. The gas sample changed from one state with a set of P-T-V-n conditions to another state with a different set of a set of P-T-V-n conditions.
The equation P•V = k describes the inversely proportional between the two quantities. But it is often more useful to express the relationship as a state equation. A state equation relates the variable values at one state to the variable values for another state after a change has occurred. Through a collection of sequential and logical steps, the P•V = k equation can be converted to a state equation. This is shown below:
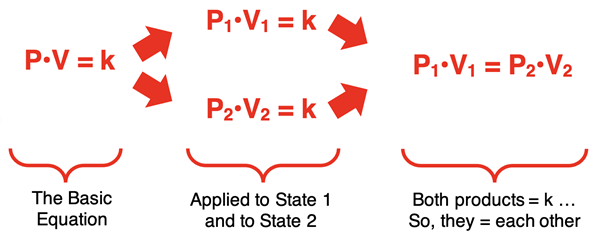
In the last step, a state equation is generated that relates the volume and pressure in one state (V1 and P1) to the volume and pressure of a second state (V2 and P2). This is one of the forms in which Boyle’s law is commonly stated.

This state equation is often used in the solution of gas law problems. Here are two examples with accompanying solutions. Additional examples are provided in the Check Your Understanding section.
Example 1
Perry O'Dictable, a close friend of Irishman Robert Boyle, measures the volume of air inside a canister to be 3.54 L when 1.38 atm of pressure is applied. If the pressure is increased to 3.99 atm, the new volume will be _____ L.
Solution:
We recommend that you begin by writing down the given values, equating them with the appropriate symbol. Then identify the unknown value. Finally, substitute the numerical values into the equation and solve using proper algebra. The solution looks like this:
Given:
V1 = 3.54 L
P1 = 1.38 atm
P2 = 3.99 atm
Unknown: V2 = ???
Equation: P1 • V1 = P2 • V2
Rearrange equation to solve for V2: V2 = P1 • V1 / P2
Substitute values into the equation: V2 = (1.38 atm) • (3.54 L) / (3.99 atm)
Use your calculator to solve: V2 = 1.22 L (rounded from 1.22436 …)
Example 2
A 55.0-L oxygen tank is filled with oxygen gas at a pressure of 7.14 atm. The tank is connected by some tubing and valves to a smaller empty tank having a volume of 35.0 L. The valves are opened, allowing some oxygen to expand into the second tank. Once the expansion has ceased, what is the final pressure? Assume a constant temperature.
Solution:
Given:
V1 = 55.0 L
P1 = 7.14 atm
V2 = 90.0 L
(NOTE: the gas expands to fill both containers - 55.0 L + 35.0 L)
Unknown: P2 = ???
Equation: P1 • V1 = P2 • V2
Rearrange equation to solve for P2: P2 = P1 • V1 / V2
Substitute values into the equation: P2 = (7.14 atm) • (55.0 L) / (90.0 L)
Use your calculator to solve: P2 = 4.36 atm (rounded from 4.363333 …)
Conclusion
We now have covered three gas laws in Lesson 2 - one for pressure and temperature, one for volume and temperature, and now one for pressure and volume. Next up is the volume and the number of moles. But before you move onward, please check out our learning suggestions below.
Before You Leave
- Download our Study Card on Pressure and Volume. Save it to a safe location and use it as a review tool.
- Try our Pressure and Volume Concept Builder with great conceptual practice on the inversely proportional relationship between these two quantities.
- Problem Set GL5 in our Calculator Pad section provides six awesome practice problems. Visit Pressure and Volume.
- The Check Your Understanding section below include questions with answers and explanations. It provides a great chance to self-assess your understanding.
Check Your Understanding
Use the following questions to assess your understanding. Tap the Check Answer buttons when ready.
 1. Consider the table at the right in answering the questions.
1. Consider the table at the right in answering the questions.
- Identify at least two sets of two rows that illustrate that a doubling of the volume will halve the pressure.
- Complete the sentence: Rows 1 and 4 illustrate that increasing the volume by a factor of ______ will cause the pressure to __________ (increase, decrease) by a factor of _____.
- Predict the value of the pressure when the volume is 16.0 L.
- Predict the value of the pressure when the volume is 24.0 L.
- Predict the value of the pressure when the volume is 20.0 L.
2. Which change would cause the pressure of a gas sample to increase?
- Increase the volume of the gas.
- Decrease the volume of the gas.
3. Starting with the state equation P
1•V
1 = P
2•V
2, use algebra to write it in four different algebraic forms. Generate a V
1 =, a V
2 =, a P
1 =, and a P
2 = equation.
4. A book is placed on top of a syringe in a chemistry lab. The volume of air in the syringe is 25.1 mL when the pressure is 5.1 psi. What volume would the air occupy when the pressure is increased to 17.9 psi?
5. A gas has a volume of 681 mL at a pressure of 745 mm Hg. What would be the pressure when the volume is 325 mL?