Hold down the T key for 3 seconds to activate the audio accessibility mode, at which point you can click the K key to pause and resume audio. Useful for the Check Your Understanding and See Answers.
Lesson 2: Covalent Bonding
Part e: Formal Charge Considerations
Part 2a:
Covalent Bonds
Part 2b:
Lewis Electron Dot Structures
Part 2c:
Double and Triple Bonds
Part 2d:
Octet Breakers
Part 2e: Formal Charge Considerations
Non-Equivalent Lewis Diagrams
It is often possible to construct more than one legitimate electron dot diagram for the same molecule. Each diagram includes the proper number of electrons and satisfies the octet rule for all atoms. In Lesson 2c, we presented two possible Lewis electron dot diagrams for carbon dioxide (CO2).

In such instances, how do you evaluate the possible diagrams and make a judgement about which is the most reasonable representation of the bonding in the molecule? The conventional answer involves the use of the concept of formal charge.
Formal Charge
The concept of formal charge is designed as a means of evaluating Lewis diagrams. A formal charge is calculated for each atom in two (or more) competing diagrams. A decision as to which diagram is most reasonable is made based upon the formal charge calculations. Since we don’t expect covalently bonded atoms in molecules to be charged, the diagram that is most reasonable is the one that has formal charges of 0 for all atoms or at least the lowest charge values. For polyatomic ions, all formal charge values should add up to the charge on the ion. We would expect that the atom in the polyatomic ion that is most electronegative is the atom that has the negative formal charge value.
Formal charge can be defined as the charge that an atom would have if all the shared electrons in a molecule were shared equally among bonded atoms. For any atom in a molecule (or polyatomic ion), the formal charge value (FC) is calculated by the following formula:
FC = X – Y
where
- X = the number of valence electrons in the free atom (e.g., 4 for C, 5 for N, 6 for O, etc.)
- Y = the number of electrons in all the lone pairs + one-half the number of shared electrons.
Formal charge is a logical guide for evaluating competing electron dot diagrams. It does not give the most accurate answer all the time. But in the absence of any perfect system for evaluating competing diagrams, formal charge considerations is the best horse in the race.
Example: CO2
Let’s use the formal charge concept to evaluate the two possible electron diagrams for CO
2. The two elements are C and O. The value of X (number of valence electron in the atom) for C is 4 and the value of X for O is 6. The value of Y is determined from the structure by counting all unshared electrons and adding one-half of the shared electrons. The work is shown below.
Based on the formal charge evaluation of these two diagrams, we would have to conclude that Diagram A is the most reasonable choice. Every atom has a formal charge of 0 in Diagram A.
Example: BCl3
We discussed boron as a common exception to the octet rule on the previous page. We presented the electron dot diagram shown below in Diagram A. But many may have been thinking that Diagram B would be a more reasonable choice since it provided boron an octet of electrons. Let’s evaluate the two diagrams using formal charge considerations. The work is shown below. The calculated formal charges are shown in parenthesis next to each atom.
Based solely on a formal charge analysis, Diagram A is a more reasonable option for a Lewis electron dot diagram.
Example: SO42- Ion
Now we will analyze a
polyatomic ion. Since the ion is charged, there should be some atoms within the ion that have a formal charge. And the sum of all the formal charges should add to -2 (the charge on the ion). Finally, we would expect oxygen, the more electronegative element, to have any negative formal charges.
Two possible electron dot diagrams are shown. Both cases are reasonable. Diagram B assumes
an expanded octet on the central sulfur atom. The formal charge calculations are shown. The calculated formal charges are shown in parenthesis next to each atom.
Based on the formal charge analysis, we would expect that Diagram B would be the more reasonable diagram. Three of the five atoms in Diagram B have zero formal charge and the other two atoms have a formal charge of -1. For an ion with a charge of 2-, that is what we expect. For Diagram A, all atoms have a formal charge. Using formal charge evaluations, we always pick the diagram that has the smallest formal charges.
Before You Leave
- Download our Study Card on Formal Charge. Save it to a safe location and use it as a review tool.
- The Check Your Understanding section below include questions with answers and explanations. It provides a great chance to self-assess your understanding.
Check Your Understanding
Use the following questions to assess your understanding. Tap the Check Answer buttons when ready.
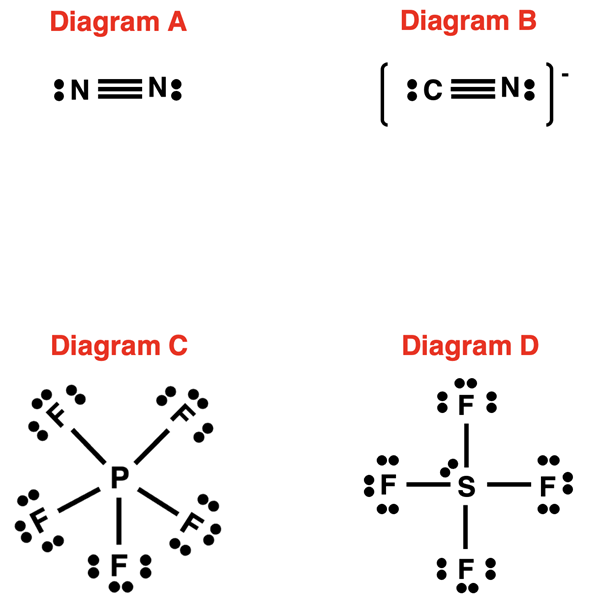
1. Calculate the formal charges on the atoms in each of the following diagrams:
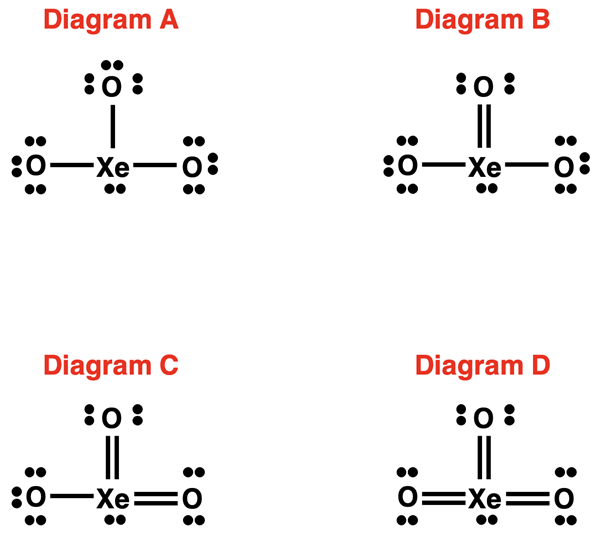
2. Examine the four possible Lewis electron dot diagrams for XeO3. Based on formal charge considerations, which one is the most reasonable diagram? Show all your work and explain the rationale behind your answer.
 3. Consider the SO42- ion discussed earlier on this page. Conduct a formal charge analysis for the electron dot diagram shown at the right. Conduct a formal charge analysis for this diagram and explain why this would not be more reasonable than Diagram B selected above.
3. Consider the SO42- ion discussed earlier on this page. Conduct a formal charge analysis for the electron dot diagram shown at the right. Conduct a formal charge analysis for this diagram and explain why this would not be more reasonable than Diagram B selected above.