Hold down the T key for 3 seconds to activate the audio accessibility mode, at which point you can click the K key to pause and resume audio. Useful for the Check Your Understanding and See Answers.
Lesson 3: Electrons
Part c: Exceptions to the Rule
Part 3a:
Electron Configurations
Part 3b:
Electrons and the Periodic Table
Part 3c: Exceptions to the Rule
Aufbau Principle
 A fundamental principle of the quantum mechanical model is that electrons must completely fill the orbitals of an energy sublevel before an electron can enter the next highest energy sublevel. The principle is known as the Aufbau Principle. The principle would assert that a ground state electron cannot reside in a 2p orbital until the 1s orbital and the 2s orbital are first filled with their allowed two electrons. Thus, we only observe electrons entering 2p orbitals for atoms containing at least five electrons. Similarly, an electron will not enter a 3p orbital until the 1s (2 e-s), 2s (2 e-s), 2p (6 e-s), and 3s (2 e-s) orbitals are completely filled. As such, an atom must have at least 13 electrons before an electron enters a 3p orbital. This Aufbau Principle is one of three principles used in determining the order in which electrons fill the orbitals of the various energy sublevels. Hund’s Rule and the Pauli Exclusion Principle are the other two.
A fundamental principle of the quantum mechanical model is that electrons must completely fill the orbitals of an energy sublevel before an electron can enter the next highest energy sublevel. The principle is known as the Aufbau Principle. The principle would assert that a ground state electron cannot reside in a 2p orbital until the 1s orbital and the 2s orbital are first filled with their allowed two electrons. Thus, we only observe electrons entering 2p orbitals for atoms containing at least five electrons. Similarly, an electron will not enter a 3p orbital until the 1s (2 e-s), 2s (2 e-s), 2p (6 e-s), and 3s (2 e-s) orbitals are completely filled. As such, an atom must have at least 13 electrons before an electron enters a 3p orbital. This Aufbau Principle is one of three principles used in determining the order in which electrons fill the orbitals of the various energy sublevels. Hund’s Rule and the Pauli Exclusion Principle are the other two.
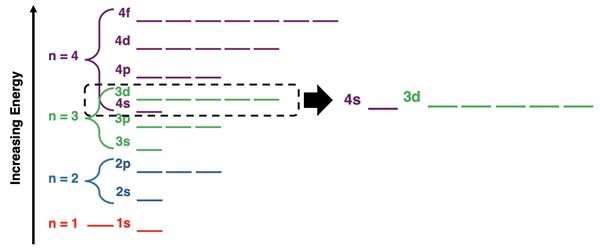
Relative Energies of 4s and 3d Orbitals
We discussed the relative energy levels of the various energy sublevels in Lesson 2c. We mentioned that the 4s orbitals are slightly lower in energy than the 3d orbitals. An energy level diagram like the one below on the left was presented. The vertical spacing provided between energy levels on the diagram makes it a useful tool for drawing “electron arrows” in the blanks to show how electrons enter the orbitals. However, the spacing also can leave the false impression that the 4s orbitals are significantly lower in energy than the 3d orbitals. The fact is that these two sets of energy sublevels are very close to one another. The diagram shown on the right provides a more accurate depiction of the close proximity of the 4s sublevel and the slightly higher energy 3d sublevel. Understanding this close proximity will be helpful in understanding the topic on this page – the exceptions to the Aufbau Principle.
Chromium and Copper as Exceptions to the Rules
The two most commonly mentioned exceptions to the Aufbau Principle are the elements chromium (24Cr) and copper (29Cu). Following the Aufbau Principle, we would expect:
24Cr: [Ar] 4s2 3d4
29Cu: [Ar] 4s2 3d9
But we experimentally observe that both chromium and copper have a half-filled s orbital. Chromium has all its d orbitals half-filled, and copper has its d orbitals completely filled. The observed arrangement of electrons is:
24Cr: [Ar] 4s1 3d5
29Cu: [Ar] 4s1 3d10
How can this be explained? Atoms of transition metals gain some added stability in half-filling or completely filling all d orbitals. This added stability is enough to cause a
promotion of an electron from the slightly lower energy 4s orbital to a 3d orbital in order to half-fill or fully fill all five d orbitals. The fact that the other exceptions to the Aufbau Principle occur for identical reasons gives some credibility to the explanation.
Keep in mind that this is an exception to the rule and not a new rule that is to be accepted. The exceptions are primarily observed with atoms of chromium and copper and a few other situations.
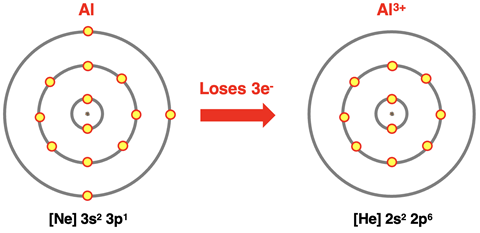
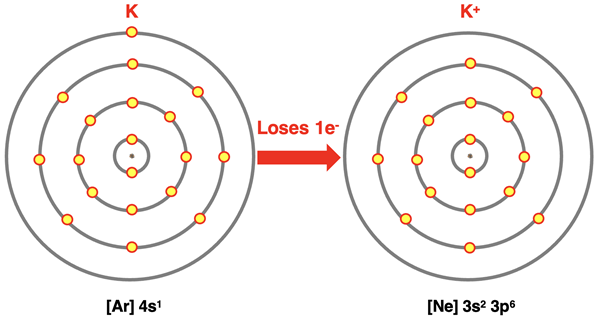
Ions of Main Group Elements
We learned in Chapter 3 that the main group metals formed positive ions by losing electrons. From an atomic structure perspective, main group metals lose their valence shell electrons. In doing so, they obtain the electron configuration of a noble gas. Aluminum (13Al) loses three electrons (two s orbital electrons and one p orbital electron) to become like neon. Calcium (20Ca) loses two electrons (both s orbital electrons) to become like argon. Potassium (19K) loses one s orbital electron to become like argon. Noble gases have a very stable electron configuration. By becoming like a noble gas, metal ions achieve stability. We can represent this process using electron shell diagrams and electron configurations.

Transition Metal Ions as Exceptions to the Rules
We first discussed transition metal ions in Chapter 3. They are quite different than main group elements in that there are multiple possible charges that they can assume. For instance, an iron atom can become the Fe2+ or the Fe3+ ion. A copper atom can become the Cu+ or the Cu2+ ion. While there are a few transition metals that form only one ion, the general tendency is for them to be multivalent.
When we discussed the process of writing electron configurations for ions, we mentioned that the first step is to determine the total number of electrons. Then proceed in the usual manner for filling orbitals with adherence to the Aufbau Principle, Hund’s Rule, and the Pauli Exclusion Principle. The process works for main group elements. It does not work for the transition metals. Transition metal atoms in the fourth period have electrons in the 4s and the 3d orbitals. When they become ions, the first electrons to be lost are the 4s electrons. Once both 4s electrons are lost, any additional loss of electrons will come from the 3d orbitals. Here are the electron configurations for several transition metal ions from the fourth period:
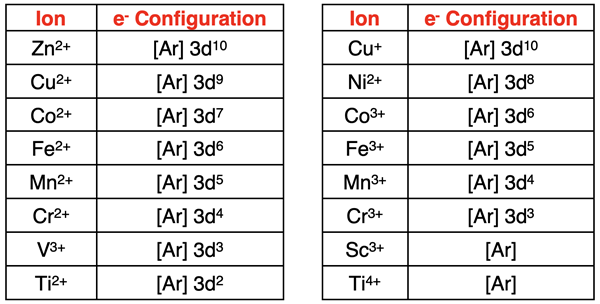
Like main group metals, transition metals become ions by losing valence shell electrons. The valence shell electrons are the 4s electrons. A transition metal will typically empty its 4s orbitals. They are said to acquire a pseudo noble gas configuration. Like a noble gas, they have a set of s and p orbitals that are full of electrons. But unlike a noble gas, transition metal ions also have some 3d electrons in their valence shell.
Before You Leave
- The Check Your Understanding section below include questions with answers and explanations. It provides a great chance to self-assess your understanding.
Check Your Understanding
Use the following questions to assess your understanding. Tap the Check Answer buttons when ready.
1. Based on what you have learned on this page, what would you predict the abbreviated electron configuration to be for molybedenum (42Mb) and silver (47Ag)?
2. Write the abbreviated electron configuration for the following transition metal ions:
NOTE: The atomic number is provided to help in locating the element on the periodic table.
- 24Cr6+
- 46Pd2+
- 48Cd2+
- 73Ta5+
- 78Pt2+
- 79Au3+
3. The element tin (50Sn) most often forms a 4+ ion. Suggest an abbreviated electron configuration for tin.