Hold down the T key for 3 seconds to activate the audio accessibility mode, at which point you can click the K key to pause and resume audio. Useful for the Check Your Understanding and See Answers.
Lesson 1: Physics in the Early 20th Century
Part a: Emission Spectrum of the Elements
Part 1a: Emission Spectrum of the Elements
Part 1b: The Photon
Part 1c: Bohr's Quantized Energy Levels
Part 1d: Wave-Particle Duality
Physics in the Early 20th Century
Probing the inner workings of an invisible atom is a challenge. The basic strategies in the early 20th century involved either bombarding an object with a small particle or energizing the atom by heating, application of high voltage, or illumination with light. Observing how the atom responds to bombardment or energizing allows scientists to determine the contents and structure of this black box we call the atom.
In this chapter we will see how scientists arrived at the modern model of the atom. It’s a story that involves many individuals and many discoveries. As it often goes in science, one discovery leads to another discovery which in turn leads to another discovery. The journey to understanding the atom’s contents and structure highlights the nature of science as a cooperative venture. Scientists work together and share ideas in an effort to understand their world. In the early 20th century, it was the work of several physicists studying the interaction between light and matter that led to the emergence of quantum mechanics.
Electromagnetic Waves
 Since the mid-1860s, it was believed that light was a wave with fluctuating electric and magnetic properties. These wave-like fluctuations come in a range of frequencies and result in varying wavelengths. The term electromagnetic wave was used to describe light and the wide range of frequencies and wavelengths was termed the electromagnetic spectrum. The electromagnetic spectrum was divided into regions, many of which may be familiar to you, like radio waves, microwaves, infrared waves, visible light waves, ultraviolet waves, x-rays, and gamma rays. The graphic below shows these regions, ordered from lowest frequency (left side) to highest frequency (right side) waves. Since wavelength is inversely proportional to frequency, the higher frequency regions of the spectrum are the shorter wavelength regions. The relative length of the waves for the various regions are depicted at the bottom of the graphic by comparing the wavelength to the size of a familiar object – like a building, a housefly, or an atom.
Since the mid-1860s, it was believed that light was a wave with fluctuating electric and magnetic properties. These wave-like fluctuations come in a range of frequencies and result in varying wavelengths. The term electromagnetic wave was used to describe light and the wide range of frequencies and wavelengths was termed the electromagnetic spectrum. The electromagnetic spectrum was divided into regions, many of which may be familiar to you, like radio waves, microwaves, infrared waves, visible light waves, ultraviolet waves, x-rays, and gamma rays. The graphic below shows these regions, ordered from lowest frequency (left side) to highest frequency (right side) waves. Since wavelength is inversely proportional to frequency, the higher frequency regions of the spectrum are the shorter wavelength regions. The relative length of the waves for the various regions are depicted at the bottom of the graphic by comparing the wavelength to the size of a familiar object – like a building, a housefly, or an atom.
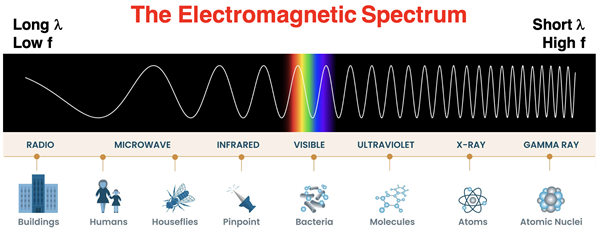
Source: NASA (public domain)
Visible Light Waves
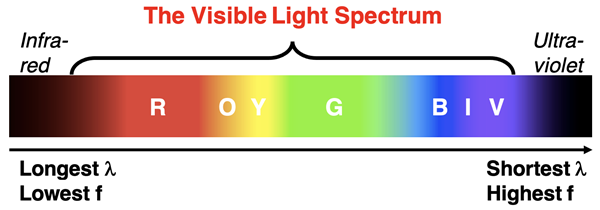
Special instruments are required to detect most of the regions of the electromagnetic spectrum. The human eye is capable of detecting a very small sliver of the electromagnetic spectrum. This sliver of the spectrum is referred to as the Visible Light region. Visible light waves consist of waves with wavelength ranging from about 400 nanometers to 700 nanometers. (A nanometer is one-billionth of a meter.)
Each wavelength of visible light is associated with a perceivable color. Wavelengths around 660 nm are perceived as red light while wavelengths of 460 nm are perceived as blue light. The visible light region is subdivided into regions based on color. Those sub-regions can be remembered by the mnemonic ROY G BIV. Each letter of Roy’s name represents a color – red, orange, yellow, green, blue, indigo, and violet. The red corresponds to the longest waves and the violet corresponds to the shortest waves.
Concepts of Continuous versus Quantized
The electromagnetic and visible light spectra are examples of continuous spectra. Referring to something as being continuous is the opposite of referring to something as being quantized. The opposing concepts of continuous and  quantized was a common scientific theme during the early 20th century. We will encounter the concepts frequently throughout this chapter.
quantized was a common scientific theme during the early 20th century. We will encounter the concepts frequently throughout this chapter.
Continuous infers that something can have any value within a range of values. The electromagnetic spectrum is referred to as continuous because waves can have any frequency or wavelength value between the bottom and the top ends of the range of values. There is a smooth and uninterrupted progression in wavelength from the radio wave to the gamma ray regions. A ramp built from the ground to the top of a building is a good analogy of the concept of continuous. A person could be standing at any location along the ramp at any time. As such, the person can assume any height between ground height and building top height. There is a continuous range of possible height values.
 Quantized infers that something can have only certain allowed values within the range of values. Instead of every value within the range being possible, quantized means that only distinct values are possible. Progression from the lowest end of the range to the highest end of the range occurs in distinct steps or jumps from one allowed value to the next allowed value. A ladder stretching from ground level to the top of a building is a good analogy of the concept of being quantized. Progression from the ground to the building top occurs in steps. At any given time, a person can only be on a rung of the ladder and not at any location in between. As such, the person assumes only certain allowed heights between ground height and building top height. There is not a continuous range of height values, but only certain allowed height values that are determined by the ladder rungs.
Quantized infers that something can have only certain allowed values within the range of values. Instead of every value within the range being possible, quantized means that only distinct values are possible. Progression from the lowest end of the range to the highest end of the range occurs in distinct steps or jumps from one allowed value to the next allowed value. A ladder stretching from ground level to the top of a building is a good analogy of the concept of being quantized. Progression from the ground to the building top occurs in steps. At any given time, a person can only be on a rung of the ladder and not at any location in between. As such, the person assumes only certain allowed heights between ground height and building top height. There is not a continuous range of height values, but only certain allowed height values that are determined by the ladder rungs.
Hydrogen Line Spectrum
When a sample of hydrogen gas is heated or if an electric voltage is impressed across it, the hydrogen gas glows with a pink color. Light is being emitted by the energized atoms of hydrogen.

The color pink that our eye-brain system perceives is actually the result of four distinct wavelengths of light being released by the glowing hydrogen. Four distinct colored lines can be perceived if the light is viewed through a spectroscope. The spectroscope separates the light of varying wavelengths and we observe a line spectrum. The actual wavelengths of the lines are predictably known to be 410 nm, 434 nm, 486 nm, and 656 nm.
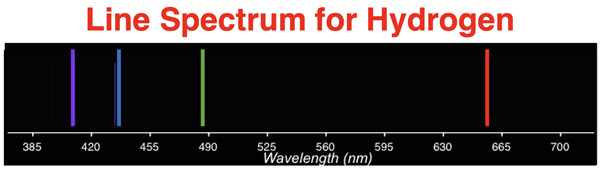
This line spectra is unique to hydrogen. Other elements have their own line spectrum. In fact, the line spectra becomes an identifying mark of an element. It is like an elemental fingerprint. But all line spectra are non-continuous. There is not a smooth and continuous progression of wavelengths and colors. The fact that only certain wavelengths of light are emitted by the energized gas indicates that it is the result of something that is quantized.
Electron Transitions
In the early 20th century, it was believed that the emission spectra of elements was caused by electrons within the atoms undergoing changes in energy levels. Electrons typically exist in the ground state. The ground state is the lowest energy state of an electron. When a sample of atoms is energized by application of heat or electricity, the electrons absorb the energy and assume an excited state. Excited states are unstable, higher energy levels that electrons can assume. The jump upward in energy from the ground state to an excited state occurs when the electron absorbs the energy. Since exited states are unstable, an excited electron will eventually jump downward in energy until it reaches the stable ground state. These downward jumps in energy result in the release of light energy.
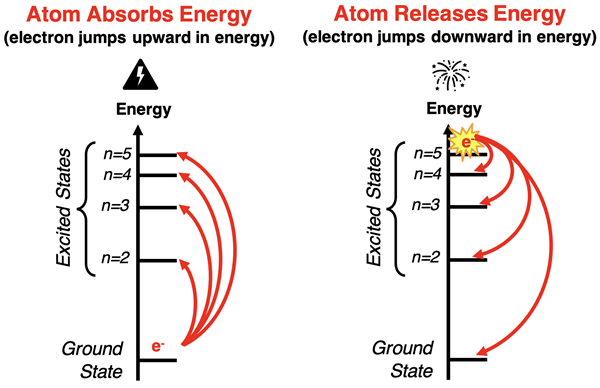
The fact that the emission spectrum of elements are line spectrum and not continuous spectrum is telling. Energy states of electrons must be quantized. There can only be certain energies that the electrons of an atom can have. The actual set of allowed energies is dependent upon the element. But all elements have electrons with quantized energy levels.
Exactly why would this be? And what can explain the actual wavelength values observed in the hydrogen line spectrum and the spectra of other elements? How can the wavelength of an emission spectrum be related to the energy states of the electrons? These were the questions that would have to be answered on the journey towards the understanding of the atom. That journey continues on the next page as we look at the contributions of physicists like Max Planck and Albert Einstein.
Before You Leave
- At some point during this lesson, you should try our Line Spectra Concept Builder. This might be a good time to get started on it.
- The Check Your Understanding section below include questions with answers and explanations. It provides a great chance to self-assess your understanding.
Check Your Understanding
Use the following questions to assess your understanding. Tap the Check Answer buttons when ready.
1. Rank the following four regions of the EM Spectrum in order of their frequencies from lowest to highest:
X-ray, microwave, ultraviolet, visible light.
2. Rank the following four regions of the EM Spectrum in order of their wavelengths from shortest to longest:
gamma ray, infrared, radio, visible light.
3. Rank the following colors of the Visible Light region in order of their frequencies from lowest to highest:
green, orange, red, blue.
4. Rank the following colors of the Visible Light region in order of their wavelengths from shortest to longest:
yellow, red, violet, blue.
5. Tom claims to be low maintenance. When he adds sweetener to his coffee, he adds it in packets – 1 packet, 2 packets, 3 packets, but never in-between. Becky on the other hand is more refined in her coffee-sweetening habits. She adds exactly the amount of sweetener that the coffee requires. This means she may add 1.05 packets, or 1.7 packets, or 1.582 packets. Write a sentence and use the words
quantized and
continuous to describe Tom and Becky’s quite different coffee-sweetening habits. Explain your descriptions.
6. Complete this sentence with the words higher and lower:
When atoms absorb energy, electrons will jump from a _______ to a _______ energy level.
7. Complete this sentence with the words higher and lower:
When electrons jump from a _______ to a _______ energy level, light energy is released.
8. Besides the four wavelengths of light emitted by energized hydrogen gas, there are also wavelengths of 121 nm and 1005 nm (and many others) emitted. Propose a reason for why these wavelengths are not seen in the line spectra of hydrogen.