Hold down the T key for 3 seconds to activate the audio accessibility mode, at which point you can click the K key to pause and resume audio. Useful for the Check Your Understanding and See Answers.
Lesson 2: Chemistry as a Quantitative Science
Part c: Quantities and Their Meaning
Part 2a: Scientific Notation
Part 2b: Units and the Metric System
Part 2c: Quantities and Their Meaning
Quantities in Chemistry
 Earlier in this lesson, the seven base quantities and units that form the foundation of the Metric System of measurement were introduced. These quantities and their units can be combined in various ways to form derived quantities. Since Chemistry is a quantitative science, a good deal of time will be devoted to understanding the various quantities of Chemistry and their relationship to one another. Of the seven base or fundamental quantities, the three that will be regularly used and integrated throughout the course are Mass, Amount of Substance, and Temperature. Length and Time will be used primarily to form derived quantities. Electric current and luminous intensity will be more thoroughly studied in other science courses (e.g., Physics).
Earlier in this lesson, the seven base quantities and units that form the foundation of the Metric System of measurement were introduced. These quantities and their units can be combined in various ways to form derived quantities. Since Chemistry is a quantitative science, a good deal of time will be devoted to understanding the various quantities of Chemistry and their relationship to one another. Of the seven base or fundamental quantities, the three that will be regularly used and integrated throughout the course are Mass, Amount of Substance, and Temperature. Length and Time will be used primarily to form derived quantities. Electric current and luminous intensity will be more thoroughly studied in other science courses (e.g., Physics).
The goal of this part of Lesson 2 is to discuss the meaning of the most commonly used base and derived quantities. This page will represent an introduction to these quantities. They will each be revisited in significantly more detail in later chapters of the Tutorial.
Mass
The quantity mass provides a measure of how much stuff is present in a sample of matter. It is commonly measured and expressed in grams or kilograms. The mass of a sample of matter depends on how many atoms are in that sample of matter and what types of atoms are in that sample. We will revisit this concept in more detail as we begin to discuss elements and the Periodic Table. For now, simply think of mass as having to do with the amount of stuff. The more stuff (i.e., atoms) an object has, the more mass that it has.
Mass is often confused with the quantity that we know as weight. Mass and weight are related but they are NOT equivalent terms. In fact, they are two distinctly different quantities. While mass refers to the amount of stuff present in an object, weight is the force of gravitational attraction of that stuff towards Earth. Weight is a force. Specifically, it is the force of gravity acting upon the stuff of an object. The confusion arises in Chemistry class because we often place objects on electronic balances to determine their mass. The process of doing so is referred to as weighing. But the result of the process is not a determination of the weight of the object but a determination  of its mass. Technically, the electronic balance determines how much the object weighs then uses gravitational information to calculate and display the object’s mass. To help (we hope) with much of the confusion, we will often use an unconventional term (i.e., we made it up) for this process – massing. Massing, as we use the term, refers to the process of determining the mass of a sample of matter by placing it on an electronic balance.
of its mass. Technically, the electronic balance determines how much the object weighs then uses gravitational information to calculate and display the object’s mass. To help (we hope) with much of the confusion, we will often use an unconventional term (i.e., we made it up) for this process – massing. Massing, as we use the term, refers to the process of determining the mass of a sample of matter by placing it on an electronic balance.
Amount of Substance
There are many ways to express how much of a substance is present in a sample of matter. Mass, volume, weight, area (in some i nstances) are all ways of referring to how much substance there is. In the metric system, amount of substance refers to the number of things. The things could be atoms or molecules or particles or distinct units of the substance. We are referring to a counting amount. It is comparable to counting the amount (i.e., number) of donuts in the box or the amount (i.e., number) of students in the school. We are literally referring to the count. Chemists (and Chemistry students) count using the metric unit known as the mole. We will save the rest of the fun regarding the mole for Chapter 7 of our Tutorial.
nstances) are all ways of referring to how much substance there is. In the metric system, amount of substance refers to the number of things. The things could be atoms or molecules or particles or distinct units of the substance. We are referring to a counting amount. It is comparable to counting the amount (i.e., number) of donuts in the box or the amount (i.e., number) of students in the school. We are literally referring to the count. Chemists (and Chemistry students) count using the metric unit known as the mole. We will save the rest of the fun regarding the mole for Chapter 7 of our Tutorial.
Temperature
Put simply, temperature is a measure of how hot or how cold a sample of matter is. Even more simply put, temperature is whatever the thermometer reads. But to put it more scientifically, temperature is a measure of how fast, on average, the particles of a sample of matter are moving or vibrating or rotating. In this sense, a thermometer that measures temperature is acting as a speedometer that measures the energy of motion of the particles of the sample. High temperature samples of matter contain particles that are in motion with lots of energy.
We are jumping ahead of ourselves a bit (more like, a lot). But while we are on the subject, it is a good time to point out an important principle. Often times in Chemistry, the observations that we make reveal something more fundamental about matter at the particle level – on the level of atoms and molecules. The macroscopic observations – the things we observe - are indicators of a particle-level or particulate realities. Go back to the  last period and read that sentence again and try to digest it … because it's a BIG idea and a deep idea. The principle is that there is often a relationship between the observables and the particles. The thermometer reading a high value is an observable; and it means the particles in the sample are moving with lots of energy. You will see this macroscopic-particulate principle in action again and again throughout your Chemistry class.
last period and read that sentence again and try to digest it … because it's a BIG idea and a deep idea. The principle is that there is often a relationship between the observables and the particles. The thermometer reading a high value is an observable; and it means the particles in the sample are moving with lots of energy. You will see this macroscopic-particulate principle in action again and again throughout your Chemistry class.
Now I think we were talking about temperature. Temperature is a base metric quantity with the base unit Kelvin (abbreviated K and having no degree symbol). Two other temperature scales that you might be familiar with are the Celsius scale and the Fahrenheit scale. The Celsius scale is a metric scale that is closely related to the Kelvin temperature scale. The Fahrenheit scale is a non-metric scale. For both the Kelvin and the Celsius scale, there are 100 temperature divisions between the freezing point of water and the boiling part of water. That is, on both scales, the boiling point of water is 100 units greater than the freezing point. A 1-unit division on the Kelvin scale is equal to a 1-unit division on the Celsius scale. And a 10 K increase in temperature corresponds to a 10°C increase in temperature. The difference between the Kelvin scale and the Celsius scale pertains to the zero point of the scale. A temperature of 0 K corresponds to a temperature of -273.15 °C. This temperature is known as the absolute temperature. At 0 K, particles stop moving. (There it is again – the macroscopic-particulate connection.)
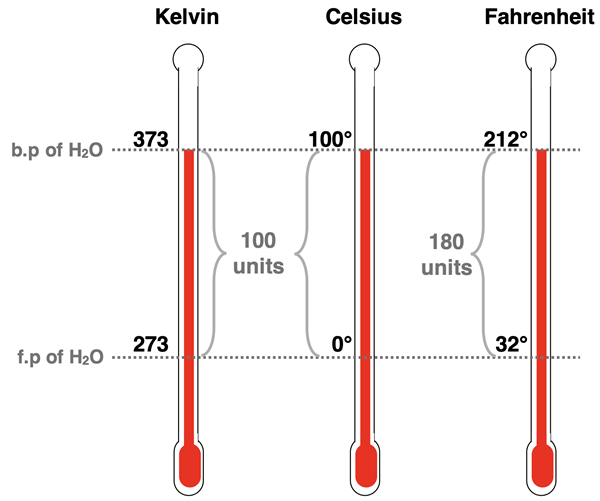
An equation can be written to relate at Kelvin temperature to a Celsius temperature. It would look like this:
Kelvin Temperature = Celsius Temperature + 273.15
And by algebraic re-arrangement:
Celsius Temperature = Kelvin Temperature - 273.15
The Fahrenheit to Celsius temperature conversions must consider two differences – a zero point difference and a difference between the size of a 1-unit division on each scale. On the Fahrenheit scale, there are 180 divisions between the freezing point of water and the boiling part of water. And so, a division on the Fahrenheit scale is 1.8 times smaller than on the Celsius scale. Furthermore, a temperature of 0 °C (freezing point of water) is equivalent to a temperature of 32°F. And so, the equations relating Celsius and Fahrenheit temperatures are:
Fahrenheit Temperature = Celsius Temperature * 1.8 + 32
Celsius Temperature = (Fahrenheit Temperature – 32) ÷ 1.8
Volume
Volume refers to the amount of space occupied by an object or a sample of matter. Volume is not a base quantity. It is a derived quantity. To illustrate consider a sample of matter that has the shape of a rectangular cuboid. A cuboid is like a cube, but with unequal length sides. From geometry, we know that the volume of a cuboid is given by the equation …
For a cuboid: Volume = Height * Width * Depth
 Since the volume is determined by multiplying base quantities, the length of each side, it is a derived quantity. Each of these three dimensions would be expressed by the base unit meter. Determining a volume of a cuboid using the above equation would result in a numeric value that has units of meter * meter * meter. In other words, a unit of volume would be meter cubed or m3.
Since the volume is determined by multiplying base quantities, the length of each side, it is a derived quantity. Each of these three dimensions would be expressed by the base unit meter. Determining a volume of a cuboid using the above equation would result in a numeric value that has units of meter * meter * meter. In other words, a unit of volume would be meter cubed or m3.
In Chemistry, we will be measuring the volume of liquids and gases that do not have a fixed shape of a cuboid. And we will measure the volume of solids that do have a fixed shape, but not that of a cuboid or any other regularly shaped object. The above formula is not useful in such cases. Instruments known as graduated cylinders will commonly be used to measure volumes. Depending on the size of the cylinder, the volume unit will be in liter or milliliter. The relationship between liter and cubic meter is
1 m3 = 1000 liter.
And Many More
Besides the above – mass, amount of substance, temperature, and volume – there are many other quantities in Chemistry that deserve a discussion. These include derived quantities like pressure, density, molarity, and rate. But we are going to save some fun for later chapters and postpone a detailed discussion of these quantities until later. It just wouldn’t be right to do all the fun stuff in the first chapter and not have anything left for later. Sorry … you’re going to have to wait.