Hold down the T key for 3 seconds to activate the audio accessibility mode, at which point you can click the K key to pause and resume audio. Useful for the Check Your Understanding and See Answers.
Lesson 3: LeChatelier's Principle
Part b: Predicting the Direction of Shift
Part a:
Disturbances and Stress
Part b: Predicting the Direction of Shift
LeChatelier’s Principle
 Reversible reaction systems that have achieved an equilibrium state can be disturbed by a change in concentration, pressure, or temperature. These disturbances were discussed in Lesson 3a. A disturbance will place stress upon the system to re-establish a new equilibrium position. LeChatelier’s principle states that the system will respond in such a way as to alleviate or counteract the effects of the stress. The response is often referred to as a shift in the equilibrium. The system is said to shift to the right to produce additional products or to shift to the left to produce additional reactants. In Lesson 3b, we will be discussing these shifts and their effect upon the set of equilibrium concentrations.
Reversible reaction systems that have achieved an equilibrium state can be disturbed by a change in concentration, pressure, or temperature. These disturbances were discussed in Lesson 3a. A disturbance will place stress upon the system to re-establish a new equilibrium position. LeChatelier’s principle states that the system will respond in such a way as to alleviate or counteract the effects of the stress. The response is often referred to as a shift in the equilibrium. The system is said to shift to the right to produce additional products or to shift to the left to produce additional reactants. In Lesson 3b, we will be discussing these shifts and their effect upon the set of equilibrium concentrations.
Understanding Shifts
The fundamental nature of the equilibrium state is that Q = K. That is, the reaction quotient (Q) is equal to the equilibrium constant (K). The reaction quotient is determined by substituting concentrations of reactants and products at any given instant in time into the equilibrium expression. These concentrations have values in the initial state that are different than their values in the equilibrium state. As such, the value of Q is changing and continues to change until finally the Q value is equal to K. At that point, equilibrium is established, and concentrations no longer undergo change.
 A disturbance of an equilibrium results when either Q or K is changed. When either is changed, the condition of Q = K is no longer true; the system is not at equilibrium. The system will adjust to this stress by shifting to the right or to the left until a new set of equilibrium concentrations cause the Q value to equal the K value. A shift to the right causes product concentrations to increase and reactant concentrations to decrease. A shift to the left causes reactant concentrations to increase and product concentrations to decrease.
A disturbance of an equilibrium results when either Q or K is changed. When either is changed, the condition of Q = K is no longer true; the system is not at equilibrium. The system will adjust to this stress by shifting to the right or to the left until a new set of equilibrium concentrations cause the Q value to equal the K value. A shift to the right causes product concentrations to increase and reactant concentrations to decrease. A shift to the left causes reactant concentrations to increase and product concentrations to decrease.
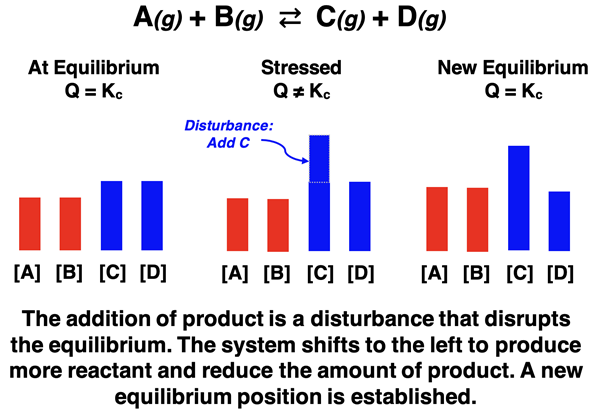
 The direction that a system shifts in response to a disturbance or stress can be reasoned through by thinking in terms of LeChatelier’s principle. The system always attempts to alleviate the stress. If a concentration of a reactant or a product is increased, the system responds with a shift in a direction that decreases it. If a pressure is increased, the system responds with a shift in a direction that reduces the overall pressure. In the sections that follow, we will discuss the details of each type of stress response and provide numerous examples of how an equilibrium shift alleviates the stress.
The direction that a system shifts in response to a disturbance or stress can be reasoned through by thinking in terms of LeChatelier’s principle. The system always attempts to alleviate the stress. If a concentration of a reactant or a product is increased, the system responds with a shift in a direction that decreases it. If a pressure is increased, the system responds with a shift in a direction that reduces the overall pressure. In the sections that follow, we will discuss the details of each type of stress response and provide numerous examples of how an equilibrium shift alleviates the stress.
 Addition of Reactants or Products
Addition of Reactants or Products
Adding reactants or products to a system is a disturbance that disrupts the equilibrium state. The system responds with a shift that alleviates the stress. If a reactant is added to the system, the system shifts to the right in the direction of the forward reaction. This reduces the concentrations of the reactants and increases the concentration of products, thus alleviating the stress caused by the addition of reactant. Similarly, if a product is added to the system, the the system shifts to the left in the direction of the reverse reaction. This reduces the concentrations of the products and increases the concentration of reactants, thus alleviating the stress caused by the addition of product.

Example 1:
For the reversible system: H2(g) + I2(g) ⇄ 2 HI(g), in what direction will the equilibrium shift if H2 gas is added? And how will the shift affect the concentrations of I2 and HI?
Example 2:
For the reversible system: H2O(g) + CO(g) ⇄ H2(g) + CO2(g), in what direction will the equilibrium shift if H2 gas is added? And how will the shift affect the concentrations of CO and CO2?
Example 3:
For the reversible system: Cl2(g) + 2 Br-(aq) ⇄ Br2(/) + 2 Cl-(aq), in what direction will the equilibrium shift if Br2 is added? And how will the shift affect the concentrations of Cl2 and Cl-?
There is an underlying reason for these directional shifts that is associated with the mechanics of a reversible system. At equilibrium, the rate of a forward reaction equals the rate of the reverse reaction. Both of these rates are dependent upon concentration. Adding a reactant to a system at equilibrium, increases the rate of the forward reaction. The forward and reverse reaction rate are no longer equal. Since the forward reaction occurs faster than the reverse reaction, the immediate response of the system is to begin increasing product concentration. These changes continue until the rates are once again equal and equilibrium is established.

Removal of Reactants or Products
Removing a reactant or product from a system is another disturbance that disrupts the equilibrium state. The system responds with a shift that alleviates the stress. If a reactant is removed from the system, the the system shifts to the left in the direction of the reverse reaction. This increases the concentrations of the reactants and decreases the concentration of products, thus alleviate the stress caused by the removal of reactant. Similarly, if a product is removed from the system, the the system shifts to the right in the direction of the forward reaction. This increases the concentrations of the products and decreases the concentration of reactants, thus alleviate the stress caused by the addition of product.

Example 4:
For the reversible system: H2(g) + I2(g) ⇄ 2 HI(g), in what direction will the equilibrium shift if H2 gas is removed? And how will the shift affect the concentrations of I2 and HI?
Example 5:
For the reversible system: H2O(g) + CO(g) ⇄ H2(g) + CO2(g), in what direction will the equilibrium shift if H2 gas is removed? And how will the shift affect the concentrations of CO and CO2?
Changes in Pressure and Volume
As we learned in the Gases and Gas Laws Chapter of our Chemistry Tutorial, the pressure and volume of a gas are inversely related. An increase in volume will decrease the pressure of a gas. And a decrease in volume will cause an increase in pressure. Either change can alter the reaction quotient and disturb the equilibrium. Determining how the system will shift is most easily done by thinking in terms of pressure.
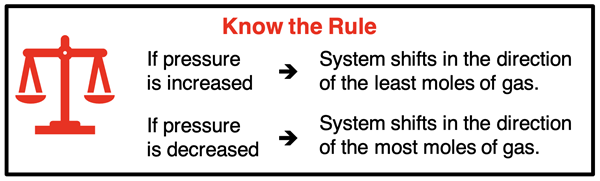
Pressure is the result of gas particles applying forces to the container walls. The more gas particles that are applying force, the greater the pressure. So, if a system at equilibrium encounters a pressure increase, the stress can be counteracted by reducing the amount of gas in the container. The system accomplishes this by shifting in the direction that produces the least moles of gas. That could be the forward direction or the reverse direction, depending on the balanced chemical equation. You can determine which direction it would be by inspecting the coefficients for any gaseous reactant or product. For the reaction,
N2(g) + 3H2(g) ⇄ 2 NH3(g),
there are four moles of gas on the reactant side and two moles of gas on the product side. A shift in the forward direction produces the least moles of gas.
If the pressure of a system is decreased (e.g., by increasing the volume), the reaction will shift in a direction that produces the most moles of gas. A shift in that direction will increase the number gas particles colliding with the container walls. With more collisions, the system will be able to counteract the stress caused by the decrease in pressure.
Example 6:
For the reversible system: 2 SO2(g) + O2(g) ⇄ 2 SO3(g), in what direction will the equilibrium shift if the pressure is increased (e.g., by decreasing the volume)? And how will the shift affect the concentrations of SO2 and SO3?
Example 7:
For the reversible system: Cl2(g) + 2 Br-(aq) ⇄ Br2(/) + 2 Cl-(aq), in what direction will the equilibrium shift if the pressure is decreased (e.g., by increasing the volume)? And how will the shift affect the concentrations of Cl2 and Cl-?
Example 8:
For the reversible system: H2(g) + I2(g) ⇄ 2 HI(g) , in what direction will the equilibrium shift if the pressure is increased (e.g., by decreasing the volume)? And how will the shift affect the concentrations of I2 and HI?
Changes in Temperature
The disturbances discussed thus far are associated with a change in the reaction quotient Q. The stress were the results of Q changing while the K remained constant. Temperature effects work differently because K values are dependent upon the temperature. A change in temperature will change the K value and cause a disturbance of the equilibrium.
The manner in which a reversible system responds to a temperature change will depend on whether the reaction is endothermic or exothermic. All reactions have an enthalpy change that is positive for an endothermic reaction and negative for an exothermic reaction. Thermochemical equations list a heat term in the equation on the reactant side for endothermic reactions and on the product side for exothermic reactions.
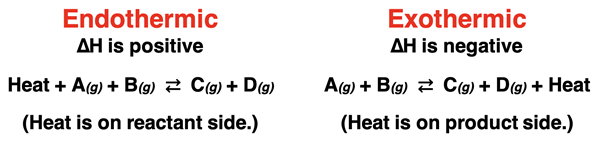
An increase in temperature can be thought of in the same way as the addition of heat. When writing a thermochemical equation, heat is identified as either a reactant or a product. An increase in temperature is equivalent to adding a reactant for an endothermic reaction and adding a product for an exothermic reaction.
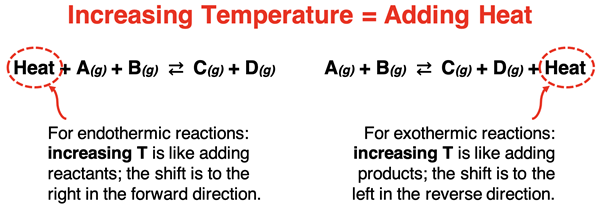
The system shifts in the direction that alleviates this addition of heat (i.e., the increase in temperature). As shown above, endothermic reactions shift in the forward direction to use up the added heat. Exothermic reactions shift in the reverse direction to use up the added heat. In both cases, the reaction shifts in the endothermic direction. For an exothermic reaction, it is the reverse direction that is endothermic. So, the easiest one-liner to describe direction shifts is that an increase in temperature causes the system to shift in the direction of the endothermic reaction.
Decreases in temperature cause the system to shift in the opposite direction as increases in temperature. A decrease in temperature causes the system to shift in the direction of the exothermic reaction.
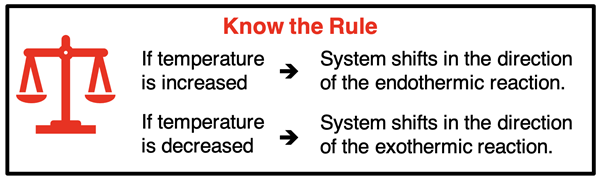
Of all the disturbances, temperature changes are the most involved. We offer the following three step approach:
1. Write the thermochemical equation with the heat term on the proper side.
2. Consider an increase in temperature as the addition of heat. Consider a decrease in temperature as the removal of heat.
3. If temperature is increased, it is like adding heat and the system shifts to react the heat away. If temperature is decreased, it is like removing heat and the system shifts in a direction to produce heat. Use this principle to decide on the direction of shift.
Example 9:
The reversible system: N2O4(g) ⇄ 2 NO2(g), has a ∆H of +57 kJ. In which direction will an increase in temperature shift the equilibriumAnd how will the shift affect the concentrations of N2O4 and NO2?
Example 10:
The reversible system: N2(g) + 3 H2(g) ⇄ 2 NH3(g), has a ∆H of -92 kJ. In which direction will a decrease in temperature shift the equilibrium? And how will the shift affect the concentrations of N2 and NH3?
Example 11:
The reversible system: N2(g) + O2(g) ⇄ 2 NO(g), has a ∆H of +66 kJ. In which direction will a decrease in temperature shift the equilibrium? And how will the shift affect the concentrations of N2 and NO?
Before You Leave
- Try our Concept Builder titled LeChatelier's Principle. Any one of the three activities provides a great follow-up to this lesson.
- The Check Your Understanding section below include questions with answers and explanations. It provides a great chance to self-assess your understanding.
- Download our Study Card on LeChatelier’s Principle. Save it to a safe location and use it as a review tool. (Coming Soon.)
Check Your Understanding
Use the following questions to assess your understanding. Tap the Check Answer buttons when ready.
1. A reversible system will encounter a shift towards the products if the rate of the forward reaction is _____ the rate of the reverse reaction.
- equal to
- greater than
- less than
2. Under what condition will the removal of products not cause a shift in the equilibrium?
- The reaction is endothermic.
- Ther reaction is exothermic.
- The number of moles of gas is the same on each side.
- The product that was removed was a solid or a liquid.
3. A system is at equilibrium. The equilibrium is then disturbed by the addition of aqueous state products. This causes the Q value to be ________ than the K value and causes a shift of the equilibrium towards the ________.
- greater, products
- greater, reactants
- less, products
- less, reactants
4. Which of the following would result from the addition of a gaseous state reactant to a system that is at equilibrium? Select all that apply.
- The value of K would change.
- The reaction quotient value (Q) would be increased by the addition of the reactant.
- The forward reaction would instantly occur at a greater rate than the reverse reaction.
- The system would shift to the left to counteract the stress.
- A new equilibrium position would eventually be achieved with increased product concentrations.
5. A reaction is exothermic. Which of the following would result from an increase in the temperature? Select all that apply.
- The value of K would change.
- The reaction quotient value (Q) would be increased by the addition of the reactant.
- The reaction would shift towards the left to counteract the increase in temperature.
- A new equilibrium position would eventually be achieved with increased product concentrations.
6. Consider the reversible system: H
2(g) + Cl
2(g) ⇄ 2 HCl
(g). The reaction is exothermic. The system is at equilibrium. Which of the following changes to the system would cause the Cl
2 concentration to increase? Select all that apply.
- HCl is removed.
- H2 Is removed.
- The temperature is increased.
- The volume of the container is increased.
- The pressure is increased.
7. Consider the reversible system: N
2(g) + 3 H
2(g) ⇄ 2 NH
3(g). The reaction is exothermic. The system is at equilibrium. Which of the following changes to the system would cause the NH
3 concentration to increase? Select all that apply.
- N2 gas is added to the system.
- H2 gas is removed from the system.
- The volume of the container is increased.
- The pressure is increased.
- The temperature is increased.
- The temperature is decreased.