Hold down the T key for 3 seconds to activate the audio accessibility mode, at which point you can click the K key to pause and resume audio. Useful for the Check Your Understanding and See Answers.
Lesson 2: Classifying Chemical Reactions
Part e: Predicting Products
Part a:
Decomposition and Synthesis Reactions
Part b:
Combustion Reactions
Part c:
Single Replacement Reactions
Part d:
Double Replacement Reactions
Part e: Predicting Products
 Reaction Types
Reaction Types
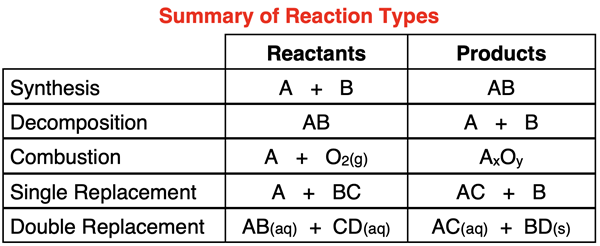
We have discussed five different reaction types in Lesson 2 with an interest in equipping a Chemistry student to predict the products of simple reactions. Synthesis and decomposition reactions were discussed in Lesson 2a. Combustion reactions were discussed in Lesson 2b. Single replacement reactions were discussed in Lesson 2c. And double replacement reactions were discussed in Lesson 2d.
Numerous examples of how to predict products and write balanced chemical equations were provided on each of these pages. Each reaction type was isolated to its own page. As such, it was relatively easy to predict products since the reaction type was obviously that which was being discussed on the particular page. Now we will see all of the reaction types mixed together on the same page. The goal is to be able to first identify the type, and then to use information about that type to predict the product. We will also write the balanced chemical equation for the reaction.
The Basic Structure of Each Reaction Type
Let’s begin with a quick review of the five reaction types. Additional details, explanations, and examples for a specific reaction type can be found by following the Learn more about … links below.
Synthesis: The reaction of two elements, an element and a compound, or two compounds to produce a single larger compound is known as a synthesis reaction. This reaction type is unique in that there is a single product. Both reactants are not necessarily free elements; but it is the only reaction type that could have two elements reacting together. The generic form for a synthesis equation is:
A + B → AB
Learn more about Synthesis Reactions.
Decomposition: In a decomposition reaction, a single compound breaks down and forms two or more smaller elements or compounds. This reaction type is unique in that there is only one reactant. The products are often (but not always) free elements. The generic form for a decomposition equation is:
AB → A + B
Learn more about Decomposition Reactions.
Combustion: In a combustion reaction, an element or a compound reacts with oxygen gas. This is sometimes referred to as burning. The products are oxides of the element(s) of the reactant. This reaction type is unique in that there are two reactants and one of them is oxygen. The generic form for a combustion equation is:
A + O2(g) → AxOy
(an element undergoes combustion)
AB + O2(g) → AwOx + ByOz
(a compound undergoes combustion)
Learn more about Combustion Reactions.
Single Replacement: In a single replacement reaction, one element replaces another element in a compound. This reaction type is unique in that there are two reactants; one is an element and the other is a compound. There are also two products – an element and a compound. The ionic compound is usually in the aqueous state. The generic form for a single replacement equation is:
AB + C → A + CB
Learn more about Single Replacement Reactions.
Double Replacement: There are two reactants in a double replacement reaction. They are both ionic compounds in the aqueous state. The cation from one compound switches places with the cation in the other compound. This reaction type is unique in that there are two reactants and they are both ionic compounds in the (aq) state. One of the products is a solid precipitate. The generic form for a double replacement equation is:
AB + CD → AD + CB
Learn more about Double Replacement Reactions.
The table below summarizes this information:
Other Prerequisite Knowledge
The skill that we are attempting to develop is: if given the reactant names or formulae, predict the product formula(e) and write a balanced chemical equation.
To accomplish this skill, there are three pieces of prior knowledge and skill you must be able to practice. Each of these prior knowledge/skill areas have been addressed on other pages of our Chemistry Tutorial. We have provided links to the relevant pages below.
- Diatomic Elements:
There are seven elements on the periodic table that exist as diatomic elements when present in their free, natural state. Those elements can be remembered by the mnemonic HONClBrIF.
Learn more about Diatomic Elements.
- Formula Writing:
A formula includes elemental symbols and subscripts. There are specific rules for writing a formula. The rules vary depending on whether the compound is ionic or molecular. Some compounds contain polyatomic ions; a list of such ions is helpful.
Learn more about Formula Writing: Ionic Compounds || Molecular Compounds
- Balancing Equations:
Chemical equations are balanced by identifying all reactant and product formulae, writing the skeleton equation, and then inserting coefficients to balance the number of atoms of each element for both sides of the equation.
Learn more about Balancing Equations.
Predicting Products and Writing Balanced Chemical Equations
For the following examples, predict the likely product(s). Then write the balanced chemical equation. Tap the
View Answer button for an answer and explanation.
Example 1
Al(s) + CuCl
2(aq)
→ ???
Example 2
NH
3(g)
→ ???
Example 3
Mg(s) + O
2(g)
→ ???
Example 4
CuCl
2(aq) + Na
3PO
4(aq)
→ ???
Example 5
CH
4(g) + O
2(g)
→ ???
Example 6
Mg(s) + S(s)
→ ???
Before You Leave
- We highly recommend our Writing Balanced Chemical Equations Concept Builder. You will get plenty of interactive practice with immediate feedback and opportunities to make corrections as you predict products and balance equations.
- The Check Your Understanding section below includes questions with answers and explanations. It provides a great chance to self-assess your understanding.
Check Your Understanding
Use the following questions to assess your understanding. Tap the
Check Answer buttons when ready.
1. Each of the following diagrams represents a reaction type. Identify each reaction type.
2. For each of the following reactions:
Classify the following reaction according to its type, predict the products, write a skeleton equation, and then write the balanced chemical equation.
- F2(aq) + CuI2(aq) →
- NH4Br(aq) + Pb(NO3)2(aq) →
- H2O(l) →
- C3H8O(s) + O2(g) →
- Al(s) + SnCl4(aq) →
- Na2CO3(aq) + Ba(NO3)2(aq) →
- S(s) + O2(g) →