Hold down the T key for 3 seconds to activate the audio accessibility mode, at which point you can click the K key to pause and resume audio. Useful for the Check Your Understanding and See Answers.
Lesson 3: Solids
Part b: Amorphous Solids
Part a:
Crystalline Solids
Part b: Amorphous Solids
Part c:
Alloys
What is an Amorphous Solid?
 We have seen earlier in Lesson 3 that crystalline solids have a highly organized arrangement of particles into a three-dimensional structure known as a crystal lattice. Not every solid is a crystalline solid. Some solids are amorphous solids. The word amorphous means without any definite shape or form. An amorphous solid is a solid that lacks a long-range order or structure of its particles.
We have seen earlier in Lesson 3 that crystalline solids have a highly organized arrangement of particles into a three-dimensional structure known as a crystal lattice. Not every solid is a crystalline solid. Some solids are amorphous solids. The word amorphous means without any definite shape or form. An amorphous solid is a solid that lacks a long-range order or structure of its particles.
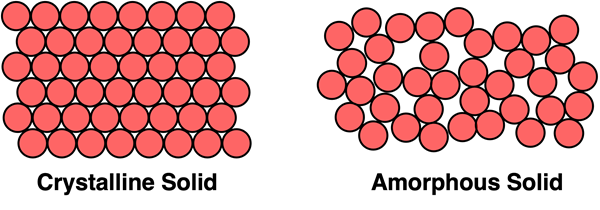
The properties of amorphous solids are considerably different than those of crystalline solids. Because of the lack of close packing of atoms, amorphous solids tend to be less dense than crystalline solids. Because of the lack of structure and reduced interparticle forces, amorphous solids tend to be brittle instead of strong. Amorphous solids are often porous, allowing liquid or air to seep in, whereas crystalline solids tend to be more impervious to surrounding fluids. And amorphous solids do not have distinct melting points. Rather than melting, they tend to progressively soften to the point of being able to flow.
Examples of amorphous solids include glass, rubber, gels, waxes, and plastics. We will discuss a few of these.
Glass
Earlier in Lesson 3, we discussed quartz, a network solid consisting of a highly organized crystal lattice of silicon and oxygen atoms. Quartz, commonly referred to as silica, has the chemical formula SiO2. If a sample of quartz is heated to high temperature so as to melt and then allowed to rapidly cool, a form of quartz known as fused quartz is produced. The structure (or lack thereof) associated with fused quartz is shown below. You will notice that the fused quartz is amorphous. It lacks the form or structure that is observed in crystalline quartz. By allowing the melted quartz to cool rapidly, it establishes a solid state before its particles are able to crystallize and assume positions within a lattice structure. The end product is an amorphous solid known as fused quartz.
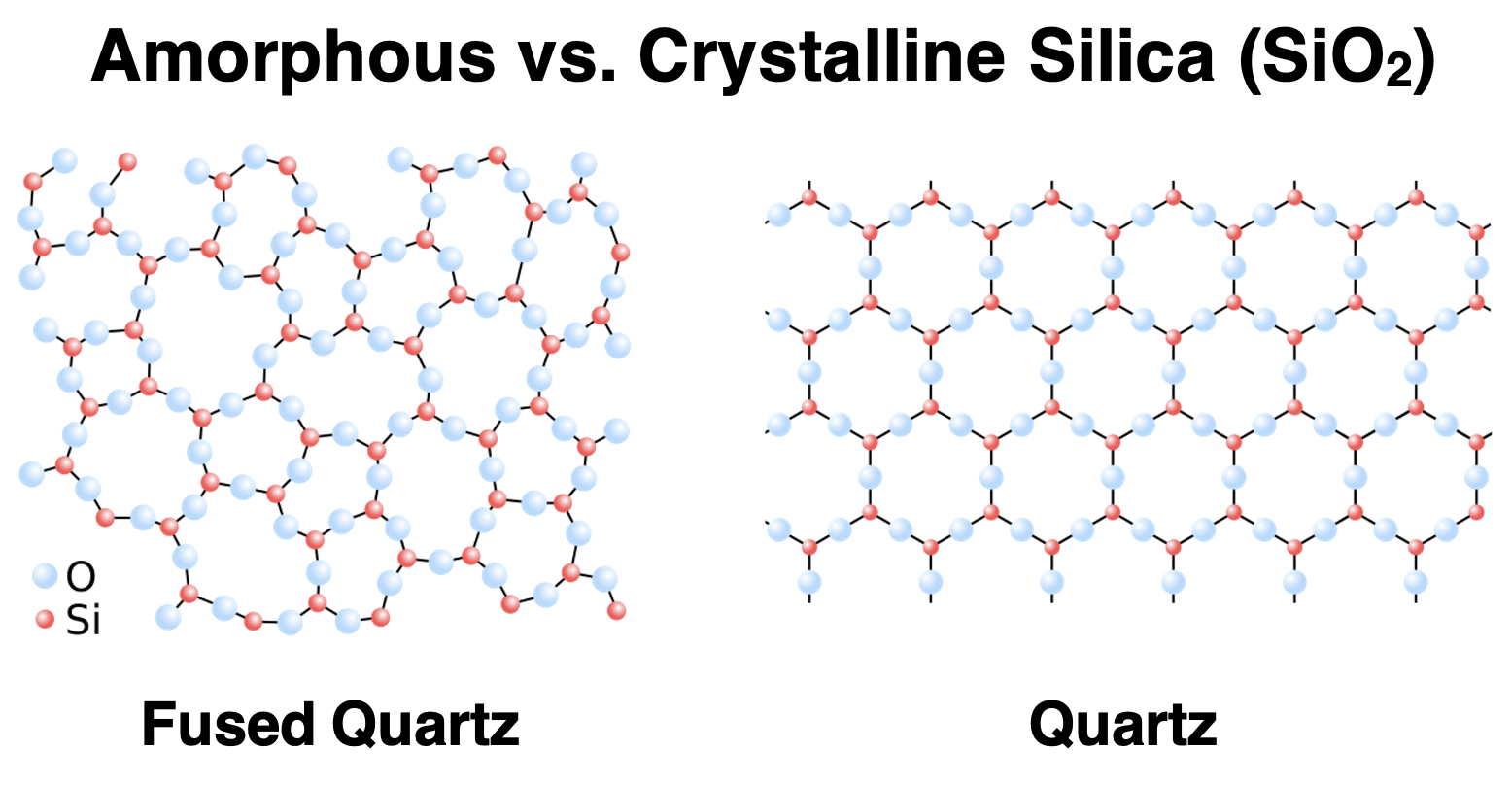
Source (Public Domain): Fused Quartz || Quartz
Glass is a manufactured material that is made in much the same way as fused quartz. It is manufactured from silica (SiO2) and other additives. Selected additives can give the glass durability, a lowered melting point, a preferred color, and increased impact resistance. The manufacturing process involves heating the silica and additives to a very high temperature (~1700°C) so that it melts. The raw materials are mixed and allowed to cool. As the glass cools, it particles become locked in a disordered state before being able to crystallize. This lack of order and structure is what makes glass an amorphous solid.
Plastics
Plastics are manufactured materials that are amorphous solids. They are a type of compound known as a polymer. A polymer is an enormously large molecule composed of relatively small repeating units linked together by covalent bonds. The repeating units are referred to as monomers.

Polyethylene is a common plastic. It is manufacturing by reacting monomer units of ethylene together to form a long (very long) chain of repeating ethylene units. A polyethylene molecule can have a chain with tens of thousands of ethylene monomers connected end to end. The actual number of ethylene monomers determines the length of the chain and the subsequent properties of the manufactured plastic. This can be controlled by modifying the temperature of the reaction, the concentration of monomers in the reactor, and the use of various chemicals that speed up or slow down the reaction.
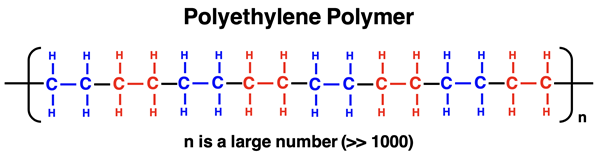
 While the structure of a polyethylene shows a highly organized collection of ethylene monomers held together by covalent bonds, the resulting polymer chains do not naturally organize themselves into a lattice. The large size of polyethylene lends itself to strong London dispersion forces. These forces hold polyethylene in the solid state. But there is still no structure assumed by the chains. They resemble a tangled mess of cooked spaghetti noodles more than the relatively organized structure of uncooked spaghetti noodles in the box.
While the structure of a polyethylene shows a highly organized collection of ethylene monomers held together by covalent bonds, the resulting polymer chains do not naturally organize themselves into a lattice. The large size of polyethylene lends itself to strong London dispersion forces. These forces hold polyethylene in the solid state. But there is still no structure assumed by the chains. They resemble a tangled mess of cooked spaghetti noodles more than the relatively organized structure of uncooked spaghetti noodles in the box.
Polyethylene has numerous uses. These uses include plastic bottles, plastic bags, flexible films, pipes and pipe fittings, and much more. There is both low-density polyethylene (LDPE) and high-density polyethylene (HDPE). The difference between the two is based on the length of the polymer chain. By controlling the chain length, chemists can control the properties of the polymer and thus the range of uses for which it can be used. And this translates into many examples of Chemistry for Better Living.
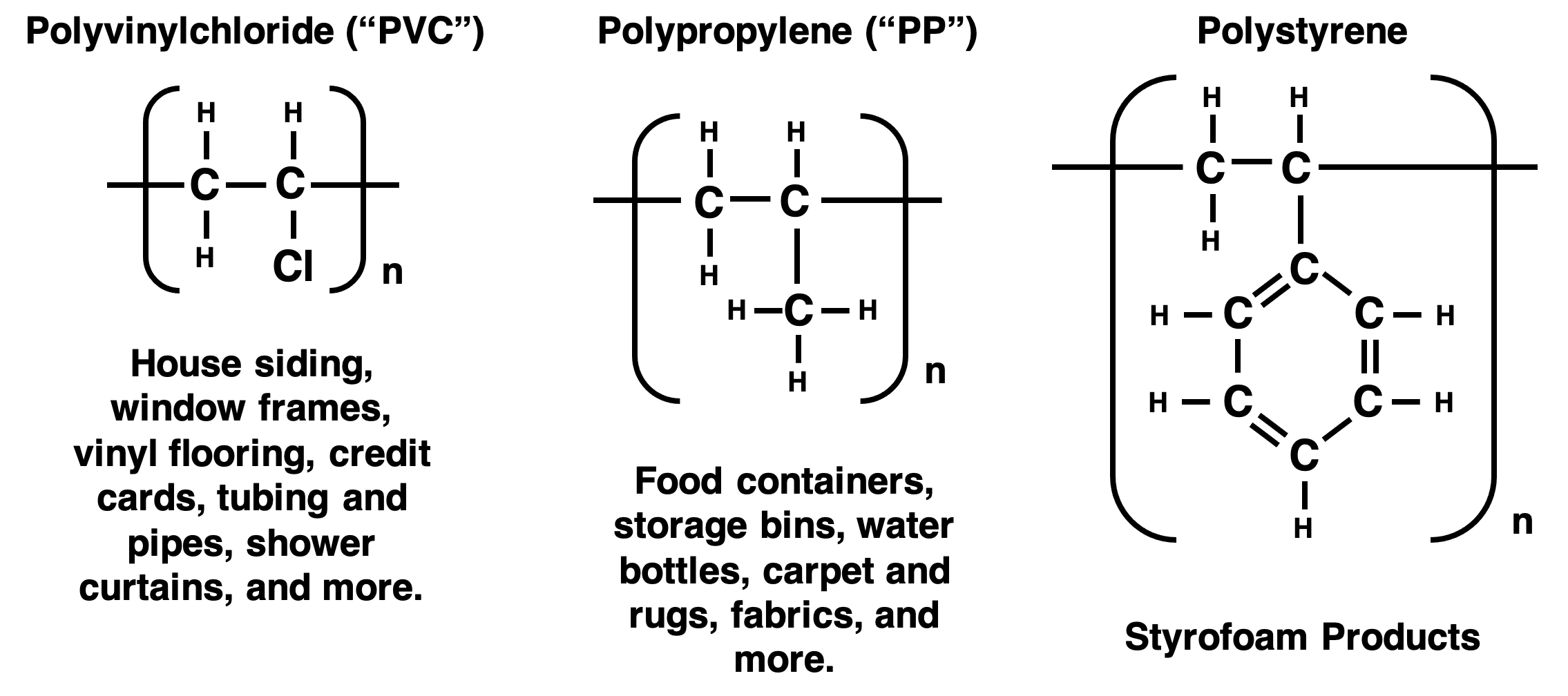
Polyethylene is just one of many polymer products that are part of our everyday lives. The list of plastics that we have come to rely on is nearly endless. Here are a few other polymers you may recognize. The repeating monomer unit that the polymer is composed and a few of their common uses are shown.
Rubber
Some polymers are composed of a single type of monomer while others may be composed of two or more monomers. Manufactured rubber is another example of an amorphous solid that is often made by combining two or more monomer units. For example, the rubber used in car tires is a polymer made by combining styrene and butadiene monomer units. An example of its structure is shown below. Chemists can control the details of the polymer structure by modifying the concentrations of the two monomers in the reactant mixture. This in turn affects the properties of the polymer product and its eventual uses.
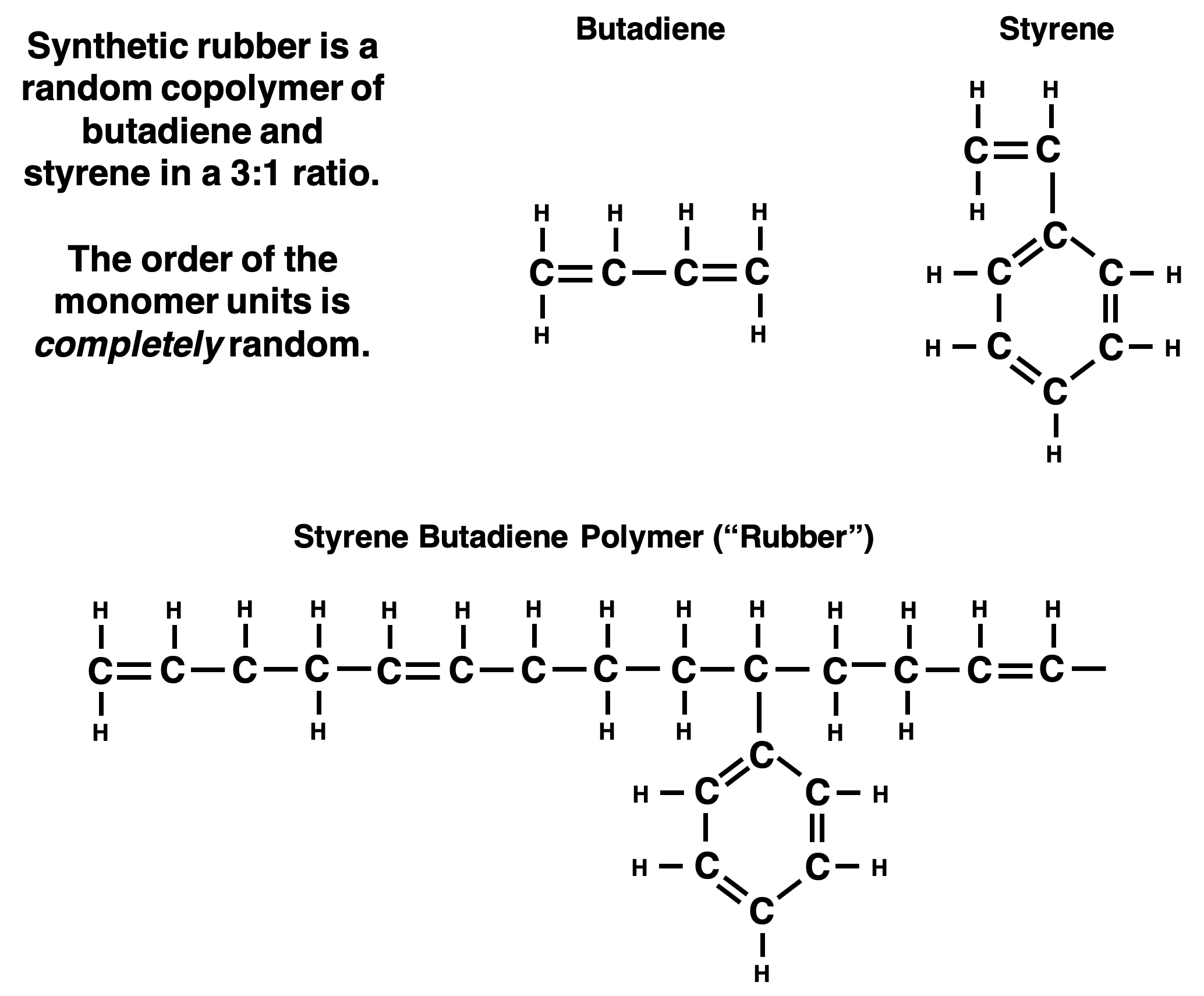
The manufacturing of rubber often includes a vulcanization step. Vulcanization involves heating the polymer with sulfur. Sulfur atoms react with the long polymer chains to create crosslinks or bridges between adjacent chains. When the polymer chain is stretched, the cross-links help the chains to return to their original orientation and shape. This gives the vulcanized rubber some resiliency and bounce.
Before You Leave
- Download our Study Card on Crystalline and Amorphous Solids. Save it to a safe location and use it as a review tool. (Coming Soon.)
- The Check Your Understanding section below include questions with answers and explanations. It provides a great chance to self-assess your understanding.
Check Your Understanding
Use the following questions to assess your understanding. Tap the Check Answer buttons when ready.
1. If an amorphous solid has no crystalline structure and no defined melting point, then what is the basis for calling it a solid?
2. Why would the strength of the interparticle forces in an amorphous solid be less than the strength observed of crystalline solids?
3. Review the structures of the polymers discussed on this page. Can you identify one characteristic that they all have in common?