Hold down the T key for 3 seconds to activate the audio accessibility mode, at which point you can click the K key to pause and resume audio. Useful for the Check Your Understanding and See Answers.
Lesson 1: Describing Chemical Reactions
Part b: Chemical Equations
Part a:
What is a Chemical Reaction?
Part b: Chemical Equations
Part c:
Writing Balanced Chemical Equations
Representations
 Managing your way through a Chemistry course can be difficult. The management process is made easier if you develop an understanding of the content presentation landscape. This landscape most often consists of verbal descriptions, particle diagrams, mathematical formulas and routines, charts and graphs, and symbolic representations of chemical systems. The variety of ways of representing chemistry concepts are intended to reinforce each other. This chapter in particular will describe chemical reactions in a variety of ways. The various parts of the landscape – particle diagrams, chemical equations (symbols), verbal descriptions, energy level diagrams (charts) - are the different ways of representing the same ideas.
Managing your way through a Chemistry course can be difficult. The management process is made easier if you develop an understanding of the content presentation landscape. This landscape most often consists of verbal descriptions, particle diagrams, mathematical formulas and routines, charts and graphs, and symbolic representations of chemical systems. The variety of ways of representing chemistry concepts are intended to reinforce each other. This chapter in particular will describe chemical reactions in a variety of ways. The various parts of the landscape – particle diagrams, chemical equations (symbols), verbal descriptions, energy level diagrams (charts) - are the different ways of representing the same ideas.
Let’s reconsider a reaction from the previous page: hydrogen gas reacts with oxygen gas to form water. Here are four means of representing this chemical reaction:

The verbal description and the particle diagram are not entirely new. But the word equation and the chemical equation may be new to your chemistry landscape. The word equation is straightforward. The chemical equation contains some details that deserve discussion.
Chemical Equations
The equation
2 H2(g) + O2(g) → 2 H2O(g)
is known as a balanced chemical equation. The balanced chemical equation uses chemical formulae (plural for formula) to identify the reactants and products of the chemical reaction. The physical state of each reactant and product is indicated with a state symbol enclosed in parenthesis and following the formula – (g) . Each formula is preceded by a number, referred to as a coefficient, that indicates the number of particles of the reactant or product involved in the reaction. If a number is not displayed in front of a formula, then the coefficient for that reactant or product is 1. Chemical equations separate the reactants from the products by an arrow (→). The arrow is a yields symbol. The arrow can be interpreted as meaning “yields”, “produces”, “reacts to produce”, etc. Reactants are located to the left of the arrow. Products are to the right of the arrow. Finally, if there is more than one reactant or more than one product, then their formulae (plural for formula) are separated by a + sign.
The collection of symbols in the chemical equation 2 H2(g) + O2(g) → 2 H2O(g) can be read to say:
Two molecules of hydrogen gas react with one molecule of oxygen gas to produce two molecules of gaseous water.
Observe that the verbal description includes information about the identity of the reactants and products, the number of particles of each, and the physical state of each.
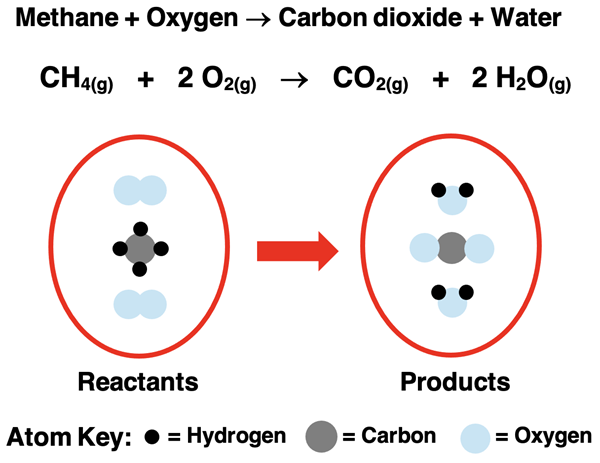
As a second example, consider the reaction of methane gas with oxygen gas that was discussed in Lesson 1a. The chemical equation is
CH4(g) + 2 O2(g) → CO2(g) + 2 H2O(g)
Take a moment to observe the formulae, the coefficients, the yields symbol, the two plus symbols, and the state symbols for each reactant and product. This collection of symbols can be read to say:
One molecule of methane gas reacts with two molecules of oxygen gas to produce one molecule of carbon dioxide gas and two molecules of gaseous water.
Law of Conservation of Mass
The coefficients that are displayed in front of the formulae are present as a book-keeping tool. As discussed in Lesson 1a, chemical reactions involve the rearrangement of atoms. The very atoms that were present in the reaction are present in the products. Atoms are not created nor destroyed in a chemical reaction. A financial accountant for a company must keep track of all the money – where it came from and where it went. In the same way, a chemical accountant (a.k.a., a chemistry student) must keep track of all the atoms. The coefficients are the means of keeping a record of the atoms to show that atoms are conserved. Such equations are referred to as balanced chemical equations.
The word equation, balanced chemical equation, and particle diagram for the reaction of methane with oxygen is shown below.
If we conduct a count of the number of atoms of the three elements for the initial condition (reactants) and the final condition (products), we will get:
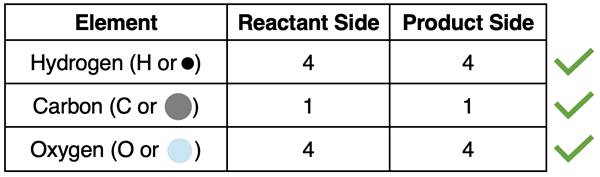
We observe that the number of atoms of each element is the same on the reactant side and the product side. That’s perfect book-keeping! The chemical equation is balanced.
The act of balancing a chemical equation is consistent with the law of conservation of mass. Atoms are conserved in a chemical reaction. And because atoms are conserved, the mass of the chemical system is also conserved. The total mass of all reactants equals the total mass of all products. The total mass of the system of reactants and produces is unchanged as the result of the reaction. When a quantity is unchanged, we refer to it as being conserved. This is one of the five postulates of John Dalton’s atomic theory discussed in Chapter 3 of this Chemistry Tutorial. We will revisit this law in Chapter 9 when we detail the mathematics of chemical reactions.
Formulae and Atom Counting
The task of making sense of symbols was introduced in Lesson 3b of Chapter 2. The skill of confidently analyzing a set of symbols in an expression like 3 CO2 + 4 H2O and determining the number of atoms of each element in the expression is critical to student success.
 A coefficient in front of a formula indicates that there are multiple copies of the formula. The symbols 3 CO2 indicate that there are three CO2 molecules. A total count of C atoms and O atoms would result in 3 C atoms and 6 O atoms.
A coefficient in front of a formula indicates that there are multiple copies of the formula. The symbols 3 CO2 indicate that there are three CO2 molecules. A total count of C atoms and O atoms would result in 3 C atoms and 6 O atoms.
 A plus sign in a formula indicates that there are additional particles – some on each side of the plus sign. The symbols 3 CO2 + 4 H2O indicate that there is a total of 7 particles – three of carbon dioxide and four of water. A total count of C atoms, O atoms, and H atoms would result in 3 C atoms, 10 O atoms, and 8 H atoms.
A plus sign in a formula indicates that there are additional particles – some on each side of the plus sign. The symbols 3 CO2 + 4 H2O indicate that there is a total of 7 particles – three of carbon dioxide and four of water. A total count of C atoms, O atoms, and H atoms would result in 3 C atoms, 10 O atoms, and 8 H atoms.
 A formula for an ionic compound sometimes includes a parenthesis with a subscripted number after it. This indicates that there are multiple copies of what is inside the parenthesis. The symbols Al2(SO4)3 indicate that there are three sulfate ions (SO4 with a 2- charge) in the formula. A total count of Al atoms, S atoms, and O atoms would result in 2 Al atoms, 3 S atoms, and 12 O atoms.
A formula for an ionic compound sometimes includes a parenthesis with a subscripted number after it. This indicates that there are multiple copies of what is inside the parenthesis. The symbols Al2(SO4)3 indicate that there are three sulfate ions (SO4 with a 2- charge) in the formula. A total count of Al atoms, S atoms, and O atoms would result in 2 Al atoms, 3 S atoms, and 12 O atoms.
In this chapter, you will learn to be a professional atom counter as you analyze chemical reactions and their symbolic representations.
Word Equations, Balanced Chemical Equations, and Particle Diagrams
As a wrap-up to Lesson 1b, three final examples of reactions with word equations, symbolic representations (chemical equations), particle diagrams, verbal interpretations, and a total atom count are presented. Study each example. Then proceed to the Before You Leave section for some suggested methods of making sense of the content of Lesson 1b.
Example 1:
Nitrogen gas reacts with hydrogen gas to produce nitrogen trihydride gas (sometimes referred to as ammonia).
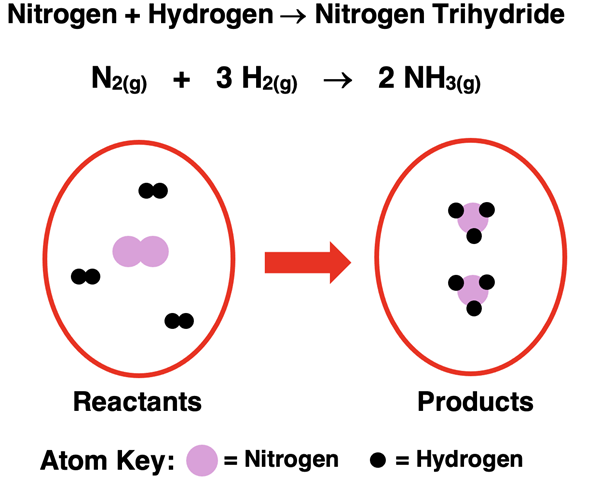
The balanced chemical equation can be interpreted to say:
One molecule of nitrogen gas reacts with three molecules of hydrogen gas to produce two molecules of nitrogen trihydride (ammonia) gas.
An atom count for nitrogen and hydrogen on the reactant and product side reveals the following totals. Because the initial number of atoms of each element is equal to the final number of atoms, it can be concluded that the chemical equation is balanced.

Example 2:
Liquid hydrogen peroxide (H2O2) decomposes to produce liquid water and oxygen gas.
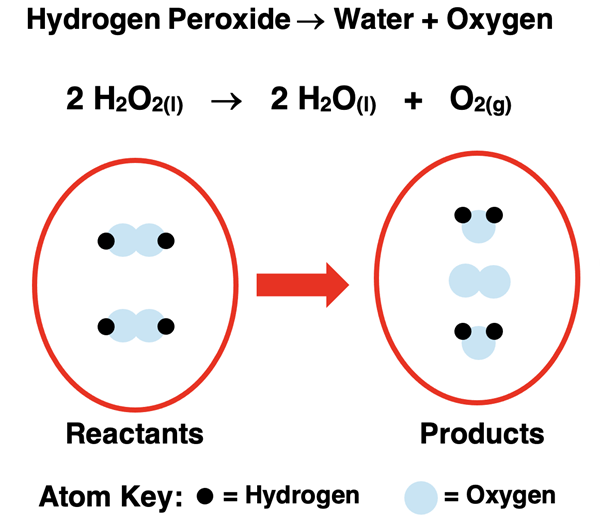
The balanced chemical equation can be interpreted to say:
Two molecules of liquid hydrogen peroxide react to produce two molecules of liquid water and one molecule of oxygen gas.
An atom count for hydrogen and oxygen on the reactant and product side reveals the following totals. Because the initial number of atoms of each element is equal to the final number of atoms, it can be concluded that the chemical equation is balanced.

Example 3:
Solid aluminum metal reacts with chlorine gas to produce solid aluminum chloride.
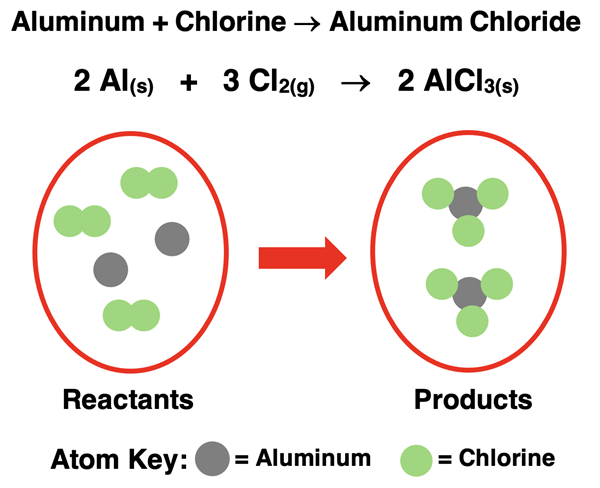
The balanced chemical equation can be interpreted to say:
Two atoms of solid aluminum react with three molecules of chlorine to produce two molecules* of solid aluminum chloride.
* Since AlCl
3 is an ionic compound, “
formula units” would be a more accurate choice than “molecules”.
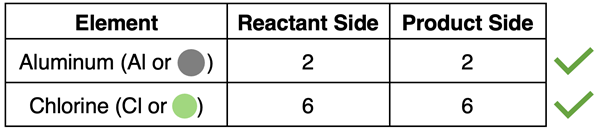
An atom count for aluminum and chlorine on the reactant and product side reveals the following totals. Because the initial number of atoms of each element is equal to the final number of atoms, it can be concluded that the chemical equation is balanced.
Before You Leave
- Download our Study Card on Chemical Equations. Save it to a safe location and use it as a review tool.
- Having troubles with atom counting? Become a pro quickly with our Formulas and Atom Counting Concept Builder. Give it a try and earn your trophy.
- The Check Your Understanding section below includes questions with answers and explanations. It provides a great chance to self-assess your understanding.
Check Your Understanding
Use the following questions to assess your understanding. Tap the
Check Answer buttons when ready.
1. Identify the following statements as being
True or
False. If False, then explain what is wrong with the statement or correct it.
- If there is no coefficient located in front of one of the formula in a balanced chemical reaction, then it is safe to assume that the equation is not yet balanced.
- A chemical equation cannot be balanced if there are no coefficients present in front of the formulae.
- The coefficients in front of the chemical formulae of a balanced chemical equation indicate the number of bonds in that particle that must be broken.
- Every balanced chemical equation has at least two formulas on the reactant side. The product side can have one or more formulas.
- When a chemical equation is not balanced, you know for certain that the reaction involves the destruction or creation of atoms.

2. Why is it important to excel towards being a professional atom counter? And where can a student acquire their professional certification?
3. A chemical equation that is not balanced is violating ___________.
- the class rules
- the 10 Commandments
- the laws of the universe
- the Law of Conservation of Mass
- the Law of Coefficient Management
4. In Example 1 of this lesson, the reactants nitrogen and hydrogen had the formulae N
2 and H
2. Why were these the formulae used? Wouldn’t N and H or even N and H
3 have been better formulae for these two reactants?
5. In Example 3 of this lesson, the reactant aluminum had the formula Al. Why was it Al and not Al
2?
6. Count the number of atoms of each element in the following set of symbols:
- 2 H2CO3 + 4 NaOH
- 2 C4H9OH + 13 O2
- 4 Al2(CO3)3
7. Provide a complete verbal interpretation of the following balanced chemical equation:
2 Na(s) + O2(g) → 2 Na2O(s)