Charge, Charge Interactions, and Charging Methods - Complete Toolkit
Objectives
- To use and understanding of atomic structure to describe how objects become charged by the additional or removal of electrons.
- To identify the type of charge on an object by observing its interactions with other charged and neutral objects.
- To describe what charge polarization is and to use an understanding of the distinction between insulators and conductors to explain how and why polarization occurs.
- To describe the charging by friction process, to explain the result of such a process, and to use such an understanding to predict the charges two objects would acquire when rubbed together.
- To describe the charging by induction process, to explain the result of such a process, and to use such an understanding to predict the charge an object would acquire when charged by induction.
- To describe the charging by conduction process, to explain the result of such a process, and to use such an understanding to predict the charge an object would acquire when charged by conduction.
- To describe the grounding process and to explain how it occurs in terms of electron movement.
Readings from The Physics Classroom Tutorial
- The Physics Classroom Tutorial, Static Electricity Chapter, Lesson 1
- The Physics Classroom Tutorial, Static Electricity Chapter, Lesson 2
Interactive Simulations
- Charging
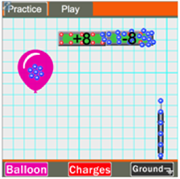 The Charging Interactive allows users to explore charge interactions, the charging of objects by conduction and induction, and the grounding of objects. This HTML5 Interactive includes two modes - Practice mode and Play mode. Practice mode is designed so that the learner can understand how objects become charged and grounded. In Practice mode, a negatively charged balloon is used to charge two conducting rods by induction. The two rods and the balloon can be dragged around the screen and the learner can explore how the arrangement of the two rods influences the manner in which they become charged. In Play mode, learners are presented with the challenge of charging each of the rods with a specific amount and type of charge. There are three different challenges; the challenges are timed and the learner's cumulative time is determined.
The Charging Interactive allows users to explore charge interactions, the charging of objects by conduction and induction, and the grounding of objects. This HTML5 Interactive includes two modes - Practice mode and Play mode. Practice mode is designed so that the learner can understand how objects become charged and grounded. In Practice mode, a negatively charged balloon is used to charge two conducting rods by induction. The two rods and the balloon can be dragged around the screen and the learner can explore how the arrangement of the two rods influences the manner in which they become charged. In Play mode, learners are presented with the challenge of charging each of the rods with a specific amount and type of charge. There are three different challenges; the challenges are timed and the learner's cumulative time is determined.
- The Concord Consortium: Modeling Electrostatics
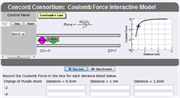 This robust, completely turn-key package features 8 interactive models to explore attraction/repulsion, Coulomb’s Law, and charge interaction at the atomic scale. Two of the models introduce math for calculating Coulomb force. Register on the website (it’s free) to access tools like Snapshot data capture and annotated photo albums for group sharing. The resource can be downloaded as a JRE file to avoid launch issues in the classroom. This activity explicitly meets NGSS Performance Expectation HS-PS2-2: Physical Science Forces & Motion – “Use mathematical representations of Coulomb’s Law to describe and predict the electrostatic forces between objects.” Don’t miss the complete lesson plan with objectives – includes assessment/answer key (link below)
This robust, completely turn-key package features 8 interactive models to explore attraction/repulsion, Coulomb’s Law, and charge interaction at the atomic scale. Two of the models introduce math for calculating Coulomb force. Register on the website (it’s free) to access tools like Snapshot data capture and annotated photo albums for group sharing. The resource can be downloaded as a JRE file to avoid launch issues in the classroom. This activity explicitly meets NGSS Performance Expectation HS-PS2-2: Physical Science Forces & Motion – “Use mathematical representations of Coulomb’s Law to describe and predict the electrostatic forces between objects.” Don’t miss the complete lesson plan with objectives – includes assessment/answer key (link below)
http://ri-itest.concord.org/SAMActivities/teacherGuides/physics/electrostatics.ver11.pdf
- Name That Charge
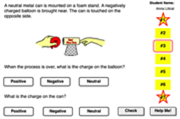 The Name That Charge Interactive is a skill building exercise that provides the learner with an interactive self-assessment of electrostatic charging methods. The Interactive presents seven different situations, provides animations of each situation, and asks the learner to identify the type of charge present on the objects as the result of the charging process. This HTML5 Interactive provides an excellent formative and self-assessment of such understandings. Charging by friction, charging by conduction (contact) and charging by induction are all targeted. Questions are pulled from a bank of 28 questions - grouped in seven groups of four questions. Both the order of the groups and the order of the questions within the group are randomized. If a student misses one of the questions from within a group, another question from within the group will be selected and delivered to the student. Questions within a group differ slightly but cover the same basic situations.
The Name That Charge Interactive is a skill building exercise that provides the learner with an interactive self-assessment of electrostatic charging methods. The Interactive presents seven different situations, provides animations of each situation, and asks the learner to identify the type of charge present on the objects as the result of the charging process. This HTML5 Interactive provides an excellent formative and self-assessment of such understandings. Charging by friction, charging by conduction (contact) and charging by induction are all targeted. Questions are pulled from a bank of 28 questions - grouped in seven groups of four questions. Both the order of the groups and the order of the questions within the group are randomized. If a student misses one of the questions from within a group, another question from within the group will be selected and delivered to the student. Questions within a group differ slightly but cover the same basic situations.
- Aluminum Can Polarization
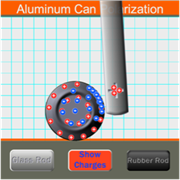 The Aluminum Can Polarization Interactive allows learners to visualize the underlying cause for the attraction between a charged and an uncharged object. The uncharged object is an aluminum can at rest on a level surface. The charged object is either a glass rod or a rubber rod. The charged object can be brought near to the uncharged object and the resulting interaction is clearly observed. But more importantly, the response of electrons within the aluminum can is depicted in the animation. A classroom-ready activity sheet is provided; the activity is open-ended and culminates with students constructing an electron density diagram in which they shade in the region of the pop can where electrons congregate most densely.
The Aluminum Can Polarization Interactive allows learners to visualize the underlying cause for the attraction between a charged and an uncharged object. The uncharged object is an aluminum can at rest on a level surface. The charged object is either a glass rod or a rubber rod. The charged object can be brought near to the uncharged object and the resulting interaction is clearly observed. But more importantly, the response of electrons within the aluminum can is depicted in the animation. A classroom-ready activity sheet is provided; the activity is open-ended and culminates with students constructing an electron density diagram in which they shade in the region of the pop can where electrons congregate most densely.
- The Concord Consortium: Atomic Structure
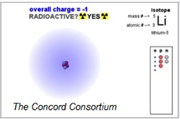 Engage in modeling atomic structures by adding or removing protons, electrons, and neutrons to construct ions and isotopes, explore the mass/charge relationship, and investigate what gives atoms charge. Every simulation is scaffolded with question sets and immediate feedback. The second section presents challenges, such as “Find a general rule for creating an atom with a stable nucleus (one that is not radioactive)”. The last set of models explores energy levels and orbitals. Why we like it: It’s pretty hard to comprehend electrical forces between atoms if you don’t understand the structure of the atom. This highly interactive resource lets students discover for themselves what causes an atom’s net charge to be positive, negative, or zero. Oh….it also meets four distinct NGSS Core Ideas – see Standards below.
Engage in modeling atomic structures by adding or removing protons, electrons, and neutrons to construct ions and isotopes, explore the mass/charge relationship, and investigate what gives atoms charge. Every simulation is scaffolded with question sets and immediate feedback. The second section presents challenges, such as “Find a general rule for creating an atom with a stable nucleus (one that is not radioactive)”. The last set of models explores energy levels and orbitals. Why we like it: It’s pretty hard to comprehend electrical forces between atoms if you don’t understand the structure of the atom. This highly interactive resource lets students discover for themselves what causes an atom’s net charge to be positive, negative, or zero. Oh….it also meets four distinct NGSS Core Ideas – see Standards below.
- PhET: Build An Atom
 Newly rewritten to HTML, this PhET simulation could be ideal for conceptual physics or a Physics First curriculum. It lets you build atoms by adding protons, electrons, and neutrons. Electrons automatically float to their appropriate orbital. Alongside the model, you can view the net charge, mass number, and where your element appears on the periodic table. After practicing with atom building, learners can test their skills against the clock in a game with 4 levels of difficulty.
Newly rewritten to HTML, this PhET simulation could be ideal for conceptual physics or a Physics First curriculum. It lets you build atoms by adding protons, electrons, and neutrons. Electrons automatically float to their appropriate orbital. Alongside the model, you can view the net charge, mass number, and where your element appears on the periodic table. After practicing with atom building, learners can test their skills against the clock in a game with 4 levels of difficulty.
Video and Animations
- MIT Electrostatics Visualizations: Creation of an Electric Dipole
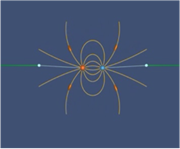 How is electric field created and how does it come to fill up space? This animation from MIT's TEAL Project starts with two opposite point charges being separated to form a classic electric dipole field configuration. It will help students see that electric fields do not appear instantly in space when there is unbalanced charge. They propagate outward from a source in a predictable way. As it begins, the positive point charge is sitting directly on top of the negative charge, so that the total charge exactly cancels out (there is no electric field). The two charges are then pulled apart, resulting in the classic dipole field pattern. As the distance between the charges increases, the strength of field decreases.
How is electric field created and how does it come to fill up space? This animation from MIT's TEAL Project starts with two opposite point charges being separated to form a classic electric dipole field configuration. It will help students see that electric fields do not appear instantly in space when there is unbalanced charge. They propagate outward from a source in a predictable way. As it begins, the positive point charge is sitting directly on top of the negative charge, so that the total charge exactly cancels out (there is no electric field). The two charges are then pulled apart, resulting in the classic dipole field pattern. As the distance between the charges increases, the strength of field decreases.
- Science Off the Sphere – Dancing Droplets
 Physics teachers have seen lots of demos on electrostatics, but we bet you haven’t seen this one! Don Pettit, an astronaut aboard the International Space Station, places a charge on knitting needles made of two substances (nylon and Teflon). Then he uses a small syringe to squirt water droplets. What happens is mind-bending. In the near absence of gravity, the water droplets curve into an orbit around the knitting needles – going slowly at first, but gaining velocity as they get closer to the charged needles. Finally, the droplets will settle on the needle’s surface. Dr. Pettit gives a great explanation of why you can see this phenomenon in space, but not on Earth.
Physics teachers have seen lots of demos on electrostatics, but we bet you haven’t seen this one! Don Pettit, an astronaut aboard the International Space Station, places a charge on knitting needles made of two substances (nylon and Teflon). Then he uses a small syringe to squirt water droplets. What happens is mind-bending. In the near absence of gravity, the water droplets curve into an orbit around the knitting needles – going slowly at first, but gaining velocity as they get closer to the charged needles. Finally, the droplets will settle on the needle’s surface. Dr. Pettit gives a great explanation of why you can see this phenomenon in space, but not on Earth.
Teachers: If students are using mobile devices, they can view the same video on YouTube (it just won’t have the supplementary information). Here’s the link:
https://www.youtube.com/watch?v=ERCioV6amys
- Physlet Physics Explorations: Electrostatics
 At The Physics Classroom, we love ranking tasks. This one from Physlet Physics presents one positively-charged moveable test object in a field with 5 fixed charges. Your task: rank the fixed charges from most negative to most positive. Warning: this is not as easy as it first seems. Encourage students to do numerous test runs before doing the ranking! Includes a pdf worksheet.
At The Physics Classroom, we love ranking tasks. This one from Physlet Physics presents one positively-charged moveable test object in a field with 5 fixed charges. Your task: rank the fixed charges from most negative to most positive. Warning: this is not as easy as it first seems. Encourage students to do numerous test runs before doing the ranking! Includes a pdf worksheet.
- Physlet Physics: Charging Objects and Static Cling
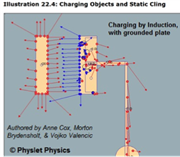 This animation set shows three ways to charge objects: friction (rubbing), induction (with and without grounding), and through “static cling”. You can view the animations in stepped motion to see more clearly how charge separation occurs, what happens to a neutral object in the polarization process, and how charge “leaks off” into a grounded plate.
This animation set shows three ways to charge objects: friction (rubbing), induction (with and without grounding), and through “static cling”. You can view the animations in stepped motion to see more clearly how charge separation occurs, what happens to a neutral object in the polarization process, and how charge “leaks off” into a grounded plate.
- “Nature” Video: Have You Ever Seen an Atom?
 At The Physics Classroom, we’ve been surprised by how many students don’t realize that microscope technology isn’t yet advanced enough to produce high-resolution images of a single atom. If they’re looking at detailed images of atomic structure, it’s a computer-generated model. But that’s changing. In 2012, physicists at UCLA figured out how to create high-resolution 3D images of platinum nanoparticles that measure only a few nanometers across. Teachers: Each of the tiny dots you see in the image above is an individual platinum atom. The research was published April 4, 2013, in Nature, Vol. 498, 74-77.
At The Physics Classroom, we’ve been surprised by how many students don’t realize that microscope technology isn’t yet advanced enough to produce high-resolution images of a single atom. If they’re looking at detailed images of atomic structure, it’s a computer-generated model. But that’s changing. In 2012, physicists at UCLA figured out how to create high-resolution 3D images of platinum nanoparticles that measure only a few nanometers across. Teachers: Each of the tiny dots you see in the image above is an individual platinum atom. The research was published April 4, 2013, in Nature, Vol. 498, 74-77.
Labs and Investigations
- The Physics Classroom, The Laboratory, Action at a Distance
Students use an understanding of the three types of charge interactions in order to determine the charge on two tapes that are charged in a different manner.
- The Physics Classroom, The Laboratory, Sticky Tape Experiments
Students rub together 10 different materials and test them to determine their charge and ultimately develop a triboelectric series.
- The Physics Classroom, The Laboratory, Pop Can Induction
Students charge two pop cans by induction using a negatively-charged balloon and determine the charge on each of the cans.
- The Physics Classroom, The Laboratory, Charging by Induction
An aluminum pie plate is charged by induction using a negatively-charged and a positively-charged object. The charge on the plate is determined for each case.
Link: http://www.physicsclassroom.com/lab#estatic
Demonstration Ideas
- University of Rochester: Electrostatics Demonstrations
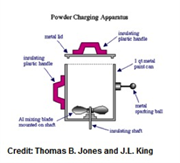 Engineering professor Thomas Jones has shared detailed “how-tos” for setting up 14 creative classroom demos on electrostatics. Most of them utilize common, inexpensive materials, with photos and diagrams to aid in set-up. You’ll be impressed with the high level of detail for building the devices and using them safely. Safety procedures are explicitly spelled out for each demo.
Engineering professor Thomas Jones has shared detailed “how-tos” for setting up 14 creative classroom demos on electrostatics. Most of them utilize common, inexpensive materials, with photos and diagrams to aid in set-up. You’ll be impressed with the high level of detail for building the devices and using them safely. Safety procedures are explicitly spelled out for each demo.
- Jefferson Lab: Static Electricity and Water
 What happens when an electrically charged object is brought near a stream of water? This video from the scientists at Jefferson Lab explains the behavior of polar molecules (such as water) in this situation. Polar molecules have an asymmetrical distribution of charge; H2O is slightly negative at the oxygen end and slightly positive at the hydrogen end. The clay model shows how the H2O molecule “flips” its positive end toward a negatively-charged PVC pipe. Very easy to set up in class.
What happens when an electrically charged object is brought near a stream of water? This video from the scientists at Jefferson Lab explains the behavior of polar molecules (such as water) in this situation. Polar molecules have an asymmetrical distribution of charge; H2O is slightly negative at the oxygen end and slightly positive at the hydrogen end. The clay model shows how the H2O molecule “flips” its positive end toward a negatively-charged PVC pipe. Very easy to set up in class.
- MIT Tech TV: Electrostatics Demo with Tinsel and Balloon
 Here’s a cool demo you can easily set up in the classroom. All you need is a Plexiglas rod, a rubber rod, animal fur for rubbing, a Mylar balloon, and holiday tinsel. Rub the rods to charge by friction. When you bring either rod close to the hanging tinsel, it flares out. Touching the tinsel with your hand discharges it. You can make the Mylar balloon move around by bringing either rod close to it. Teachers: When rubbed with the fur, the rubber rod picks up negative charge, while the Plexiglass rod picks up positive charge. If you untie the Mylar balloon, you can “chase” it around with the Plexiglass rod.
Here’s a cool demo you can easily set up in the classroom. All you need is a Plexiglas rod, a rubber rod, animal fur for rubbing, a Mylar balloon, and holiday tinsel. Rub the rods to charge by friction. When you bring either rod close to the hanging tinsel, it flares out. Touching the tinsel with your hand discharges it. You can make the Mylar balloon move around by bringing either rod close to it. Teachers: When rubbed with the fur, the rubber rod picks up negative charge, while the Plexiglass rod picks up positive charge. If you untie the Mylar balloon, you can “chase” it around with the Plexiglass rod.
Historical Connections:
- American Institute of Physics: Rutherford’s Nuclear World
 Beautifully written online exhibit explores the life and contributions of Ernest Rutherford, the dynamic New Zealander who led the team that discovered the nucleus of the atom. It presents a humanistic view of Rutherford as an energetic, good-humored man whose leadership brought out the best in his colleagues. “I remember….Geiger coming to me in great excitement and saying, ‘We have been able to get some of the a-particles coming backwards…’ It was quite the most incredible event that has ever happened to me in my life.” (E. Rutherford, 1938) The exhibit includes lots of photos, a few of Rutherford’s original drawings, rough notes from his science journals, and narration of noted physicists talking about Rutherford’s achievements.
Beautifully written online exhibit explores the life and contributions of Ernest Rutherford, the dynamic New Zealander who led the team that discovered the nucleus of the atom. It presents a humanistic view of Rutherford as an energetic, good-humored man whose leadership brought out the best in his colleagues. “I remember….Geiger coming to me in great excitement and saying, ‘We have been able to get some of the a-particles coming backwards…’ It was quite the most incredible event that has ever happened to me in my life.” (E. Rutherford, 1938) The exhibit includes lots of photos, a few of Rutherford’s original drawings, rough notes from his science journals, and narration of noted physicists talking about Rutherford’s achievements.
- How Protons, Electrons, and Neutrons were Discovered
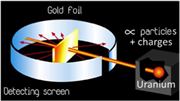 How did Thomsen, Rutherford, and Chadwick discover the electron, proton, and neutron? This video gives a very nicely illustrated overview of the scientists and the experimentation that led to these historic discoveries.
How did Thomsen, Rutherford, and Chadwick discover the electron, proton, and neutron? This video gives a very nicely illustrated overview of the scientists and the experimentation that led to these historic discoveries.
- Ben Franklin as my Lab Partner: Franklin and Electrostatics
 Chances are you haven’t seen this award-winning, comprehensive collection of resources created by Robert Morse, Fellow at the Wright Center for Science Education, Tufts University. It integrates the scientific work of Ben Franklin with 8 detailed lab procedurals for replicating the historic experiments in high school classrooms. Writings and historical observations are woven into lab descriptions to give students insight into the challenges Franklin faced as he investigated electrostatic phenomena. Includes 11 short videos in which the author explains how to set up each experiment.
Chances are you haven’t seen this award-winning, comprehensive collection of resources created by Robert Morse, Fellow at the Wright Center for Science Education, Tufts University. It integrates the scientific work of Ben Franklin with 8 detailed lab procedurals for replicating the historic experiments in high school classrooms. Writings and historical observations are woven into lab descriptions to give students insight into the challenges Franklin faced as he investigated electrostatic phenomena. Includes 11 short videos in which the author explains how to set up each experiment.
- Backstage Science: Rutherford Gold Foil Experiment
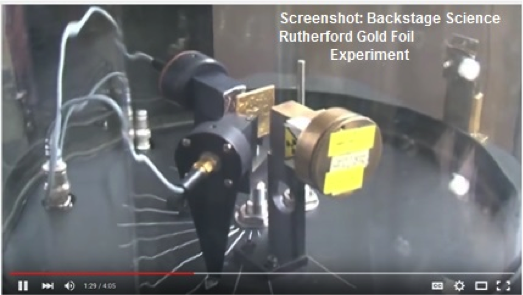 The UK’s well-known Backstage Science crew teams up with particle physicist Bruce Kennedy to reconstruct Ernest Rutherford’s historic gold foil experiment. Rutherford’s team directed beams of alpha particles through a very thin gold foil. One detector counts particles going straight ahead; another detector measures the scattering rate of particles. The experiment resulted in discovery of the atomic nucleus.
The UK’s well-known Backstage Science crew teams up with particle physicist Bruce Kennedy to reconstruct Ernest Rutherford’s historic gold foil experiment. Rutherford’s team directed beams of alpha particles through a very thin gold foil. One detector counts particles going straight ahead; another detector measures the scattering rate of particles. The experiment resulted in discovery of the atomic nucleus.
Minds On Physics Internet Modules:
The Minds On Physics Internet Modules are a collection of interactive questioning modules that target a student’s conceptual understanding. Each question is accompanied by detailed help that addresses the various components of the question.
- Static Electricity Module, Ass’t SE1 - Charges and Atoms
- Static Electricity Module, Ass’t SE2 - Interactions Between Charged Objects
- Static Electricity Module, Ass’t SE3 - Charging by Friction
- Static Electricity Module, Ass’t SE4 - Charging by Contact and the Grounding Process
- Static Electricity Module, Ass’t SE5 - Charging by Induction - Pop Can Induction
- Static Electricity Module, Ass’t SE6 - Charging by Induction - A Single Sphere
- Static Electricity Module, Ass’t SE7 - Charging by Induction - Electrophorus Plate
Concept Building Exercises:
- The Curriculum Corner, Static Electricity, Charge
- The Curriculum Corner, Static Electricity, Charge Interactions
- The Curriculum Corner, Static Electricity, Charging by Friction
- The Curriculum Corner, Static Electricity, Insulators, Conductors, and Polarizatio
- The Curriculum Corner, Static Electricity, Charging by Conduction and Grounding
- The Curriculum Corner, Static Electricity, Charging by Induction
Science Reasoning Activities:
- Charge Interactions
- Sticky Tape Experiments
Link: http://www.physicsclassroom.com/reasoning/electrostatics
Common Misconceptions:
- Proton Movement
Students will inevitably resort to explaining observed phenomenon in terms of the movement of protons. As an example, a student might assert that an object became positively-charged by gaining protons from another object or that a positively-charged object was grounded by the loss of some protons. It is important to emphasize that protons never move in electrostatic phenomenon. Take the time to review the structure of the atom, identifying the location of protons as being tightly bound within the nucleus of an atom. Provide numerous explanations of how all charging and discharging phenomenon can be explained by the addition or removal of electrons.
- The Law of Conservation of Charge
Student explanations of electrostatic phenomenon often fail to recognize the importance of the law of conservation of charge. Electrostatic phenomenon involve the transfer of electrons between two or more objects. Because the process involves transfer of negatively-charged particles, there should never be any "charge is created on Object A"-styled explanations. The fact that an object that was once neutral and is now charged negatively is simple the result of electron tranfer and not any "creation" process. It is helpful to demonstrate proper language to students when explaining phenomenon and to be vigilant at correcting students whose explanations fail to recognize charge conservation.
Elsewhere on the Web:
- NOVA ScienceNOW: Lightning
 This multimedia collection explores the electrostatic forces that cause lightning. It features a 9-minute NOVA video, an interactive tutorial on varieties of lightning, an “Ask the Expert” question/answer session with lightning expert Joe Dwyer, and background information for teachers.
This multimedia collection explores the electrostatic forces that cause lightning. It features a 9-minute NOVA video, an interactive tutorial on varieties of lightning, an “Ask the Expert” question/answer session with lightning expert Joe Dwyer, and background information for teachers.
Standards:
A. Next Generation Science Standards (NGSS)
Performance Expectations - Matter and Interactions
- HS-PS1-1: Use the periodic table as a model to predict the relative properties of elements based on the patterns of electrons in the outermost energy level of atoms.
- HS-PS1-3: Plan and conduct an investigation to gather evidence to compare the structure of substances at the bulk scale to infer the strength of electrical forces between particles.
Performance Expectations - Forces and Interactions
- HS-PS2-4: Use mathematical representations of Coulomb’s Law to describe and predict the electrostatic forces between objects.
Disciplinary Core Ideas – Matter and Interactions
- HS-PS1.A.1: Each atom has a charged substructure consisting of a nucleus, which is made of protons and neutrons, surrounded by electrons.
- HS-PS1.A.3: The structure and interactions of matter at the bulk scale are determined by electrical forces within and between atoms.
Disciplinary Core Ideas - Forces and Interactions
- HS-PS2.B.3: Attraction and repulsion between electric charges at the atomic scale explain the structure, properties, and transformations of matter, as well as the contact forces between material objects.
- HS-PS2.B.1: Newton’s Law of Universal Gravitation and Coulomb’s Law provide the mathematical models to describe and predict the effects of gravitational and electrostatic forces between distant objects.
- HS-PS2.B.2: Forces at a distance are explained by fields (gravitational, electric, and magnetic) permeating space that can transfer energy through space.
Crosscutting Concepts
Patterns
- Different patterns may be observed at each of the scales at which a system is studied and can provide evidence for causality in explanation of phenomena.
Scale, Proportion, and Quantity
- The significance of a phenomenon is dependent on the scale, proportion, and quantity at which it occurs.
- Time, space, and energy phenomena can be observed at various scales using models to study systems that are too large or too small.
Structure and Function
- The functions and properties of natural and designed objects and systems can be inferred from their overall structure, the way their components are shaped and used, and the molecular substructures of its various materials.
- Complex and microscopic structures and systems can be visualized, modeled, and used to describe how their function depends on the shapes, composition, and relationships among its parts, therefore complex natural structures/systems can be analyzed to determine how they function.
Science and Engineering Practices
Practice #2: Asking Questions and Defining Problems
- Evaluate questions that challenge the premise of an argument, the interpretation of a data set, or the suitability of a design.
Practice #3: Constructing Explanations
- Construct an explanation that includes qualitative or quantitative relationships between variables that predict phenomena.
- Construct and review an explanation based on valid and reliable evidence obtained from a variety of sources (including students’ own investigations, models, simulations, and peer review) and the assumption that theories and laws that describe the natural world operate today as they did in the past and will continue to do so in the future.
Practice #4: Developing and Using Models
- Develop and/or use a model to generate data to support explanations, analyze systems, or solve problems.
Practice #5: Engaging in Argument from Evidence
- Evaluate the claims, evidence, and reasoning behind currently accepted explanations or solutions to determine the merits of arguments.
- Make and defend a claim based on evidence about the natural world that reflects scientific knowledge and student-generated evidence.
- Construct an oral and written argument or counter-arguments based on data and evidence.
Practice #6: Planning and Carrying Out Investigations
- Plan and conduct an investigation individually and/or collaboratively to produce data to serve as the basis for evidence.
Practice #7: Using Mathematics and Computational Thinking
- Use mathematical representations of phenomena to describe explanations.
- Create or revise a simulation of a phenomenon, designed device, process, or system.
Practice #8: Obtaining, Evaluating, and Communicating Information: High School
- Evaluate the validity and reliability of multiple claims that appear in scientific and technical texts or media reports, verifying the data when possible.
- Critically read scientific literature adapted for classroom use to determine central ideas and obtain scientific/technical information.