Hold down the T key for 3 seconds to activate the audio accessibility mode, at which point you can click the K key to pause and resume audio. Useful for the Check Your Understanding and See Answers.
Lesson 2: Quantitative Analysis of Compounds
Part d: Empirical and Molecular Formulae
Part a:
Molar Mass of Compounds
Part b:
Grams-Moles-Particles Relationship
Part c:
Percent Composition
Part d: Empirical and Molecular Formulae
What’s in a Formula?
 On the previous page, the importance of the question…
On the previous page, the importance of the question…
What is the substance in this sample of matter? That is, what is its name and molecular formula?
… was discussed. A molecular formula and the composition of a compound are closely related. A molecular formula indicates which elements are present in a compound and the number of atoms of each element in a molecule of the compound. Every molecule of H
2O has two atoms of hydrogen and one atom of oxygen. On this page, we will learn how a molecular formula is determined from experimental data.
The Simplest Formula
Laboratory chemists are capable of analyzing a compound and determining the mass of each element present in the compound. Typical data from such analyses look like this:
Mass of Unknown Sample: = 10.000 g
Mass of Hydrogen in Sample = 0.593 g
Mass of Oxygen in Sample = 9.407 g
This mass information can be used to determine the moles of the two elements – H and O – in the 10.000-gram sample. By dividing by the molar mass, we can determine …
Moles of H = 0.593 g • (1 mol/1.0079 g) = 0.588 mol H
Moles of O = 9.407 g • (1 mol/15.9994 g) = 0.588 mol O
The analysis determines that the sample contains equal moles of H and O. Put another way, there are an equal number of atoms of H and O in a molecule of this compound. If you have a collection of equal numbers of H and O atoms, then there are multiple ways to assemble them into molecules. As such, the molecular formula could be HO or H
2O
2 or H
3O
3 or H
4O
4, etc. From this data alone, there is no way of knowing the molecular formula. All that is known is that the subscripts on the two elements must be identical.
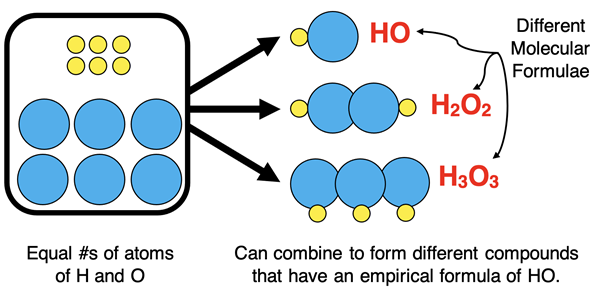
Chemists settle on what is referred to as the simplest formula. The simplest formula indicates the simplest ratio of atoms of the elements. For the above compound, the simplest formula is HO (subscripts of 1 are not included). Another name for simplest formula is empirical formula. It is referred to as an empirical formula because it is determined empirically (by experimental analysis).
Empirical Formula vs. Molecular Formula
 The empirical or simplest formula is not necessarily the molecular formula and is sometimes quite different than the molecular formula. While the empirical formula shows the simplest ratio of atoms, the molecular formula shows that actual ratio of atoms in a molecule of the compound. The subscripts on the molecular formula are always a whole number (1, 2, 3, 4, etc.) multiple of the subscripts on the empirical formula. The table below shows the molecular formula and empirical formula for several compounds.
The empirical or simplest formula is not necessarily the molecular formula and is sometimes quite different than the molecular formula. While the empirical formula shows the simplest ratio of atoms, the molecular formula shows that actual ratio of atoms in a molecule of the compound. The subscripts on the molecular formula are always a whole number (1, 2, 3, 4, etc.) multiple of the subscripts on the empirical formula. The table below shows the molecular formula and empirical formula for several compounds.
Analysis of Mass Composition Data
There are a variety of experimental methods used to determine the mass composition for the elements in an unknown compound. Our goal is to learn how to use such data and determine the empirical formula. The method is quite straightforward and based primarily on
the skill of converting from mass to moles. After several examples, we will learn how to determine a molecular formula from the empirical formula and molar mass data.
Method for Determining an Empirical Formula from Mass Data
Experimental analysis of an unknown compound will most commonly yield data in one of two formats. One format includes the mass in grams of each element in the compound. The method for determining the empirical formula from mass data is:
- For each element, use molar mass values and conversion factors to determine the # of moles of each element.
- Determine the simplest ratio of moles for all the elements. This is typically done by dividing the larger number of moles by the smaller number of moles.
- Analyze the results to determine the simplest set of whole number subscripts.

The process that takes place after step 2 can be quite varied. It takes much thoughtful practice to acquire the skill of determining the whole number subscripts from the step 2 results. Let’s talk through some examples that assume three elements (X, Y, and Z) in a compound with an empirical formula of the format X
aY
bZ
c where a, b, and c are the simplest whole number subscripts.
For Compound X
aY
bZ
c, step 2 results:
X: 1.01
Y: 1.99
Z: 1.00
The resulting numbers are close enough to whole numbers to round to 1, 2, and 1. This yields the empirical formula of X
1Y
2Z
1 or more appropriately XY
2Z.
As a second example, suppose step 2 provides the ratios …
For Compound X
aY
bZ
c:
X: 1.49
Y: 1.00
Z: 1.02
The X ratio is nowhere close to a whole number. The most reasonable approach at this point is to multiply each ratio by two. This yields new ratios of 2.98, 2.00, and 2.04. These ratios are close enough to whole numbers to conclude on an empirical formula of X
3Y
2Z
2.
As a final example, suppose step 2 provides the ratios …
For Compound X
aY
bZ
c:
X: 1.32
Y: 1.34
Z: 1.00
Once again there are one or more ratios (the X and the Y ratios) that are nowhere close to a whole number. The most reasonable approach at this point is to multiply each ratio by three. This yields new ratios of 3.96, 4.02, and 3.00. These ratios are close enough to whole numbers to conclude on an empirical formula of X
4Y
4Z
3.
Always keep in mind that you are analyzing experimental data and it is sometimes messy, and at other times, real messy. Don’t expect the analysis to yield perfect whole numbers. Errors in the experimental process are going to result in ratios like 3.96, 4.02, and 3.00 instead of the more perfect 4.00, 4.00, and 3.00. With relatively accurate data, you should be able to comfortably arrive at a set of whole number subscripts using simple multipliers of 2, 3, 4, and 5. Avoid taking ratios like 1.24, 1.01, and 1.00 and using large multipliers like 100 to end up with an empirical formula of X
124Y
101Z
100.
Examples 1 and 2 illustrate the use of these strategies.
Example 1
An analysis of a compound containing sulfur and oxygen results in the following data:
1.231 g of S
1.798 g of O
Determine the empirical formula of the compound.
Solution:
Step 1: Determine moles of each element
Sulfur: 1.231 g S • (1 mol S/32.065 g S) = 0.03839 mol S
Oxygen: 1.798 g O • (1 mol O/15.9994 g O) = 0.1123 mol O
Step 2: Determine the simplest ratio of the two elements
Sulfur: 0.03839 mol S / 0.03839 mol S = 1.00
Oxygen: 0.1123 mol O / 0.03839 mol S = 2.927
Step 3: Determine whole number subscripts for each element
The numbers 1.00 and 2.927 are close enough to whole numbers to determine that the subscripts are 1 and 3. The empirical formula is SO3.
Example 2
An analysis of a compound containing hydrogen, carbon, and oxygen results in the following data:
1.119 g of H
13.401 g of C
35.541 g of O
Determine the empirical formula of the compound.
Solution:
Step 1: Determine moles of each element
Hydrogen: 1.119 g H • (1 mol H/1.0079 g H) = 1.110 mol H
Carbon: 13.401 g C • (1 mol C/12.0107 g C) = 1.1158 mol C
Oxygen: 35.541 g O • (1 mol O/15.9994 g O) = 2.2214 mol O
Step 2: Determine the simplest ratio of the three elements
Hydrogen: 1.110 mol H / 1.110 mol H = 1.000
Carbon: 1.1158 mol C / 1.110 mol H = 1.005
Oxygen: 2.2214 mol O / 1.110 mol H = 2.001
Step 3: Determine whole number subscripts for each element
The numbers 1.000, 1.005, and 2.001 are close enough to whole numbers to determine that the subscripts are 1, 1, and 2. The empirical formula is CHO2.
Method for Determining an Empirical Formula from Percent Composition Data
Experimental analysis of an unknown compound can also yield data as percent composition (by mass) for each element in the compound. The method for determining the empirical formula from percent composition data is:
- Assume a 100.00-gram sample. Use the percent composition data to determine the mass of each element in a 100.00-gram sample.
- For each element, use molar mass values in conversion factors to determine the # of moles of each element in the 100.00-gram sample.
- Determine the simplest ratio of moles for all the elements. This is typically done by dividing the larger number of moles by the smaller number of moles.
- Analyze the results to determine the simplest set of whole number subscripts. This final analysis will proceed in an identical manner as described above.
Examples 3 and 4 illustrate the use of this method.
Example 3
Analysis of a compound containing iron and oxygen show the following percent composition by mass values: 69.945% Fe and 30.055% O. Determine the empirical formula.
Solution:
Step 1: Determine mass of each element in a 100.00-gram sample
Iron: 100.00 g • (0.69945) = 69.945 g Fe
Oxygen: 100.00 g • (0.30055) = 30.005 g O
Step 2: Determine moles of each element
Iron: 69.945 g Fe • (1 mol Fe/55.845 g Fe) = 1.2525 mol Fe
Oxygen: 30.005 g O • (1 mol O/15.9994 g O) = 1.8785 mol O
Step 3: Determine the simplest ratio of the two elements
Iron: 1.2525 mol Fe / 1.2525 mol Fe = 1.0000
Oxygen: 1.8785 mol O / 1.2525 mol Fe = 1.4998
Step 4: Determine whole number subscripts for each element
The numbers 1.00 and 1.4998 can both be multiplied by two to yield 2.0000 and 2.9997. These two numbers are close enough to whole numbers to determine that the subscripts are 2 and 3. The empirical formula is Fe2O3.
Example 4
A compound containing carbon, hydrogen, and oxygen is found to be composed of 39.999% C, 6.714% H and 53.287% O. Determine the empirical formula.
Solution:
Step 1: Determine mass of each element in a 100.00-gram sample
Carbon: 100.00 g • (0.39999) = 39.999 g C
Hydrogen: 100.00 g • (0.69945) = 6.714 g H
Oxygen: 100.00 g • (0.53287) = 53.287 g O
Step 2: Determine moles of each element
Carbon: 39.999 g C • (1 mol C/12.0107 g C) = 3.3303 mol C
Hydrogen: 6.714 g H • (1 mol H/1.0079 g H) = 6.661 mol H
Oxygen: 53.287 g O • (1 mol O/15.9994 g O) = 3.3306 mol O
Step 3: Determine the simplest ratio of the three elements
Carbon: 3.3303 mol C / 3.3303 mol C = 1.0000
Hydrogen: 6.661 mol H / 3.3303 mol C = 2.000
Oxygen: 3.3306 mol O / 3.3303 mol C = 1.0001
Step 4: Determine whole number subscripts for each element
The numbers 1.0000, 2.000, and 1.0001 are close enough to whole numbers to determine that the subscripts are 1, 2, and 1. The empirical formula is CH2O.
Determining a Molecular Formula
A molecular formula cannot be determined solely from mass composition data. A molar mass value is required to determine the molecular formula from an empirical formula. The subscripts of a molecular formula are a whole number multiple (1, 2, 3, 4, etc.) of the subscripts in the empirical formula. For this reason, the molar mass of the actual compound is also the same whole number multiple of the mass per mole of the empirical formula.
Suppose that you determine an unknown compound has an empirical formula of CH
2O. And suppose that you determine that the molar mass is 60.0 g/mol. The mass per mole of CH
2O is …
12.0107 g/mol + 2•(1.0079 g/mol) + 15.9994 g/mol = 30.00259 g/mol
The molar mass (given as 60.0 g/mol) is two times the mass per mole of CH
2O. Therefore, the subscripts in the molecular formula must be two times those of CH
2O. The molecular formula is C
2H
4O
2.
The table below demonstrates the relationship between the empirical formula, the molar mass, and the molecular formula. If you know the empirical formula and the molar mass, then you can determine the subscript multiplier and determine the molecular formula.
Before You Leave
- Download our Study Card on Empirical vs. Molecular Formulae. Save it to a safe location and use it as a review tool.
- Our Calculator Pad section has two sets of Practice Problems on this topic. Your answers are evaluated and feedback is provided; you have infinite opportunities to correct your mistakes. Try …
- The Check Your Understanding section below includes questions with answers and explanations. It provides a great chance to self-assess your understanding.
Check Your Understanding
Use the following questions to assess your understanding. Tap the
Check Answer buttons when ready.
1. A chemical formula of a compound represents ______.
- the relative mass of each element in the compound
- the relative number of each type of atom in the compound
- the relative atomic masses of each element in the compound
2. A distinction is made between the
simplest formula and the
molecular formula. The
simplest formula is sometimes referred to as the
empirical formula. What is the empirical formula of a compound which has the molecular formula of …
- … P4O10?
- … C8H18?
- … H2SO4?
3. Formulas of compounds indicate
atom ratios of the elements in the compound.
- So, the formula CO2 indicates that there is/are _____ atom(s) of C and _____ atom(s) of O in 1 molecule of CO2.
- Similarly, the formula Ca3(PO4)2 indicates that there is/are _____ atom(s) of Ca, _____ atom(s) of P, and _____ atom(s) of O in 1 molecule of Ca3(PO4)2.
4. Determine the empirical formula for a compound having the following ratio of C, N, H, and O atoms?
- 1.20 x 1021 N atoms
- 2.40 x 1021 O atoms
- 6.00 x 1021 C atoms
- 12.00 x 1021 H atoms
- C5H10O2N
- C6H12O2N
- C6H12O3N
- C6H12O24N
5. The subscripts in the molecular formula _____ the subscripts in the empirical formula.
are always the same as
- are always a whole number multiple of
- are often unrelated to
- can seldom be found from
6. A compound is analyzed and found to contain the following percentages by mass: 45.56% tin (Sn) and 54.43% chlorine (Cl). Determine the empirical formula of this compound.
7. A highly odiferous organic compound has the empirical formula CH
2 and a molar mass of 70 g/mol. Determine the molecular formula of the compound.