In an earlier section of Lesson 1, it was stated that an electrical attraction would be observed between a charged object and a neutral object. If a charged plastic tube is held near to neutral paper bits, the  attraction between the paper and the plastic would be sufficient to raise the paper off the table. If a rubber balloon is charged by rubbing it with animal fur, the balloon can subsequently be stuck to the surface of a wooden cabinet or a whiteboard. Quite surprisingly, this interaction between a neutral object and any charged object can be explained using our usual rules of opposites attract and likes repel.
attraction between the paper and the plastic would be sufficient to raise the paper off the table. If a rubber balloon is charged by rubbing it with animal fur, the balloon can subsequently be stuck to the surface of a wooden cabinet or a whiteboard. Quite surprisingly, this interaction between a neutral object and any charged object can be explained using our usual rules of opposites attract and likes repel.
Inducing the Movement of Charge
 As discussed previously, an atom consists of positively charged protons and negatively charged electrons. The protons are in the nucleus of the atom, tightly bound and incapable of movement. The electrons are located in the vast regions of space surrounding the nucleus, known as the electron shells or the electron clouds. Relative to the protons of the nucleus, these electrons are loosely bound. In conducting objects, they are so loosely bound that they may be induced into moving from one portion of the object to another portion of the object. To get an electron in a conducting object to get up and go, all that must be done is to place a charged object nearby the conducting object.
As discussed previously, an atom consists of positively charged protons and negatively charged electrons. The protons are in the nucleus of the atom, tightly bound and incapable of movement. The electrons are located in the vast regions of space surrounding the nucleus, known as the electron shells or the electron clouds. Relative to the protons of the nucleus, these electrons are loosely bound. In conducting objects, they are so loosely bound that they may be induced into moving from one portion of the object to another portion of the object. To get an electron in a conducting object to get up and go, all that must be done is to place a charged object nearby the conducting object.
To illustrate this induced movement of electrons, we will consider an aluminum pop can that is taped to a Styrofoam cup. The Styrofoam cup serves as both an insulating stand and a handle. A rubber balloon is charged negatively, perhaps by rubbing it against animal fur. If the negatively charged balloon is brought near the aluminum pop can, the electrons within the pop can will experience a repulsive force. The repulsion will be greatest for those electrons that are nearest the negatively charged balloon. Many of these electrons will be induced into moving away from the repulsive balloon. Being present within a conducting material, the electrons are free to move from atom to atom. As such, there is a mass migration of electrons from the balloon's side of the aluminum can towards the opposite side of the can. This electron movement leaves atoms on the balloon's side of the can with a shortage of electrons; they become positively charged. And the atoms on the side opposite of the can have an excess of electrons; they become negatively charged. The two sides of the aluminum pop can have opposite charges. Overall the can is electrically neutral; it's just that the positive and negative charge has been separated from each other. We say that the charge in the can has been polarized.
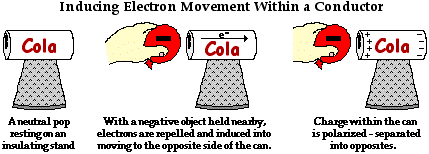
In general terms, polarization means to separate into opposites. In the political world, we often observe that a collection of people becomes polarized over some issue. For instance, we might say that the United States has become polarized over the issue of the death penalty. That is, the citizens of the United States have been separated into opposites - those who are for the death penalty and those who are against the death penalty. In the context of electricity, polarization is the process of separating opposite charges within an object. The positive charge becomes separated from the negative charge. By inducing the movement of electrons within an object, one side of the object is left with an excess of positive charge and the other side of the object is left with an excess of negative charge. Charge becomes separated into opposites.
The polarization process always involves the use of a charged object to induce electron movement or electron rearrangement. In the above diagram and accompanying discussion, electrons within a conducting object were induced into moving from the left side of the conducting can to the right side of the can. Being a conductor, electrons were capable of moving from atom to atom across the entire surface of the conductor. But what if the object being polarized is an insulator? Electrons are not free to move across the surface of an insulator. How can an insulator such as a wooden wall be polarized?
How Can an Insulator be Polarized?
Polarization can occur within insulators, but the process occurs in a different manner than it does within a conductor. In a conducting object, electrons are induced into movement across the surface of the conductor from one side of the object to the opposite side. In an insulator, electrons merely redistribute themselves within the atom or molecules nearest the outer surface of the object. To understand the electron redistribution process, it is important to take another brief excursion into the world of atoms, molecules and chemical bonds.
The electrons surrounding the nucleus of an atom are believed to be located in regions of space with specific shapes and sizes. The actual size and shape of these regions is determined by the high-powered mathematical equations common to Quantum Mechanics. Rather than being located a specific distance from the nucleus in a fixed orbit, the electrons are simply thought of as being located in regions often referred to as electron clouds. At any given moment, the electron is likely to be found at some location within the cloud. The electron clouds have varying density; the density of the cloud is considered to be greatest in the portion of the cloud where the electron has the greatest probability of being found at any given moment. And conversely, the electron cloud density is least in the regions where the electron is least likely to be found. In addition to having varying density, these electron clouds are also highly distortable. The presence of neighboring atoms with high electron affinity can distort the electron clouds around atoms. Rather than being located symmetrically about the positive nucleus, the cloud becomes asymmetrically shaped. As such, there is a polarization of the atom as the centers of positive and negative charge are no longer located in the same location. The atom is still a neutral atom; it has just become polarized.

The discussion becomes even more complex (and perhaps too complex for our purposes) when we consider molecules - combination of atoms bonded together. In molecules, atoms are bonded together as protons in one atom attract the electrons in the clouds of another atom. This electrostatic attraction results in a bond between the two atoms. Electrons are shared by the two atoms as they begin to overlap their electron clouds. If the atoms are of different types (for instance, one atom is Hydrogen and the other atom is Oxygen), then the electrons within the clouds of the two atoms are not equally  shared by the atoms. The clouds become distorted, with the electrons having the greatest probability of being found closest to the more electron-greedy atom. The bond is said to be a polar bond. The distribution of electrons within the cloud is shifted more towards one atom than towards the other atom. This is the case for the two hydrogen-oxygen bonds in the water molecule. Electrons shared by these two atoms are drawn more towards the oxygen atom than towards the hydrogen atom. Subsequently, there is a separation of charge, with oxygen having a partially negative charge and hydrogen having a partially positive charge.
shared by the atoms. The clouds become distorted, with the electrons having the greatest probability of being found closest to the more electron-greedy atom. The bond is said to be a polar bond. The distribution of electrons within the cloud is shifted more towards one atom than towards the other atom. This is the case for the two hydrogen-oxygen bonds in the water molecule. Electrons shared by these two atoms are drawn more towards the oxygen atom than towards the hydrogen atom. Subsequently, there is a separation of charge, with oxygen having a partially negative charge and hydrogen having a partially positive charge.
It is very common to observe this polarization within molecules. In molecules that have long chains of atoms bonded together, there are often several locations along the chain or near the ends of the chain that have polar bonds. This polarization leaves the molecule with areas that have a concentration of positive charges and other areas with a concentration of negative charges. This principle is utilized in the manufacture of certain commercial products that are used to reduce static cling. The centers of positive and negative charge within the product are drawn to excess charge residing on the clothes. There is a neutralization of the static charge buildup on the clothes, thus reducing their tendency to be attracted to each other. (Other products actually use a different principle. During manufacturing, a thin sheet is soaked in a solution containing positively charged ions. The sheet is tossed into the dryer with the clothes. Being saturated with positive charges, the sheet is capable of attracting excess electrons that are scuffed off of clothes during the drying cycle.)
How Does Polarization Explain the Balloon and the Wall Demonstration?
A complete discussion of the world of atoms, molecules and chemical bonds is beyond the scope of The Physics Classroom. Nonetheless, a model of the atom as a distortable cloud of negative electrons surrounding a positive nucleus becomes essential to understanding how an insulating material can be polarized. If a charged object is brought near an insulator, the charges on that object are capable of distorting the electron clouds of the insulator atoms. There is a polarization of the neutral atoms. As shown in the diagrams below, the neutral atoms of the insulator will orient themselves in such a manner as to place the more attractive charge nearest the charged object. Once polarized in this manner, opposites can now attract.

A common demonstration performed in class involved bringing a negatively charged balloon near a wooden door or wooden cabinet. The molecules of wood will reorient themselves in such a way as to place their  positive charges towards the negatively charged balloon. The distortion of their electron clouds will result in an alignment of the wood molecules in a manner that makes the wooden cabinet attracted to the negatively charged balloon. In human terms, one might say that the wood does some quick grooming and then places its most attractive side towards the balloon and its most repulsive side away from the balloon. In the world of static electricity, closeness counts. The negative balloon is closer to the positive portion of the wood molecules and further from the more repulsive negative portion. The balloon and the wall attract with sufficient force to cause the balloon to stick to the wall. From a mechanics standpoint, we would say that the balloon and the wall are pressed together with a large force. The large normal force on the balloon results in a large static friction force. This friction force balances the downward force of gravity and the balloon remains at rest.
positive charges towards the negatively charged balloon. The distortion of their electron clouds will result in an alignment of the wood molecules in a manner that makes the wooden cabinet attracted to the negatively charged balloon. In human terms, one might say that the wood does some quick grooming and then places its most attractive side towards the balloon and its most repulsive side away from the balloon. In the world of static electricity, closeness counts. The negative balloon is closer to the positive portion of the wood molecules and further from the more repulsive negative portion. The balloon and the wall attract with sufficient force to cause the balloon to stick to the wall. From a mechanics standpoint, we would say that the balloon and the wall are pressed together with a large force. The large normal force on the balloon results in a large static friction force. This friction force balances the downward force of gravity and the balloon remains at rest.
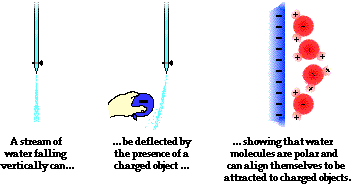
Another common physics (and chemistry) demonstration involves using a charged object to deflect a stream of water from its path. Most often, a comb is charged negatively by combing one's hair or a rubber balloon is charged in a similar manner. The negatively charged object is then brought near to a falling stream of water, causing the stream to be attracted to the comb or balloon and alter its direction of fall. The demonstration illustrates the polar nature of water molecules. The hydrogen atoms serve as the positive poles within a water molecule; oxygen serves as the negative pole. Molecules of a liquid are free to rotate and move about; the water molecules realign themselves in order to put their positive poles towards the negatively charged object. Once polarized, the stream and the balloon (or comb) are attracted. As the water molecules within the stream fall past the balloon, this realignment of individual molecules happens quickly and the entire stream is deflected from its original downward direction.
Examples of the attraction between charged objects and neutral objects are numerous and often demonstrated by physics teachers. Paper bits become polarized and are attracted to a charged piece of acetate. Small penguins cut from a sheet of paper are attracted to a charged plastic golf tube and demonstrate their happy feet. A long wooden 2x4 is placed on a pivot and becomes polarized and attracted to a charged golf tube. To the astonishment of students, the force of attraction on the wood is large enough to rotate it about the pivot point.
Polarization is Not Charging
Perhaps the biggest misconception that pertains to polarization is the belief that polarization involves the charging of an object. Polarization is not charging! When an object becomes polarized, there is 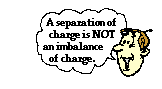 simply a redistribution of the centers of positive and negative charges within the object. Either by the movement of electrons across the surface of the object (as is the case in conductors) or through the distortion of electron clouds (as is the case in insulators), the centers of positive and negative charges become separated from each other. The atoms at one location on the object possess more protons than electrons and the atoms at another location have more electrons than protons. While there are the same number of protons and electrons within the object, these protons and electrons are not distributed in the same proportion across the object's surface. Yet, there are still equal numbers of positive charges (protons) and negative charges (electrons) within the object. While there is a separation of charge, there is NOT an imbalance of charge. When neutral objects become polarized, they are still neutral objects.
simply a redistribution of the centers of positive and negative charges within the object. Either by the movement of electrons across the surface of the object (as is the case in conductors) or through the distortion of electron clouds (as is the case in insulators), the centers of positive and negative charges become separated from each other. The atoms at one location on the object possess more protons than electrons and the atoms at another location have more electrons than protons. While there are the same number of protons and electrons within the object, these protons and electrons are not distributed in the same proportion across the object's surface. Yet, there are still equal numbers of positive charges (protons) and negative charges (electrons) within the object. While there is a separation of charge, there is NOT an imbalance of charge. When neutral objects become polarized, they are still neutral objects.
The process of polarization is often used in many charging methods. In one section of Lesson 2, the charging by induction process will be discussed. This charging process depends upon a charged object to induce polarization within a neutral object. While charging by induction includes polarization as one of its steps, polarization is still NOT a charging process. Details about the induction charging method can be read about in Lesson 2 of this unit.
We Would Like to Suggest ...

Sometimes it isn't enough to just read about it. You have to interact with it! And that's exactly what you do when you use one of The Physics Classroom's Interactives. We would like to suggest that you combine the reading of this page with the use of our
Aluminum Can Polarization Interactive. You can find it in the Physics Interactives section of our website. The
Aluminum Can Polarization Interactive assists a learner in visualizing the re-arrangement of charges within a conductor as a charged object is brought near.
Check Your Understanding
Use your understanding of charge to answer the following questions. When finished, click the button to view the answers.
1. A rubber balloon possesses a positive charge. If brought near and touched to the door of a wooden cabinet, it sticks to the door. This does not occur with an uncharged balloon. These two observations can lead one to conclude that the wall is _____.
|
a. electrically neutral
|
b. negatively charged
|
|
c. a conductor
|
d. lacking electrons
|
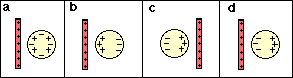
2. Which of the diagrams below best represents the charge distribution on a metal sphere when a positively charged plastic tube is placed nearby?
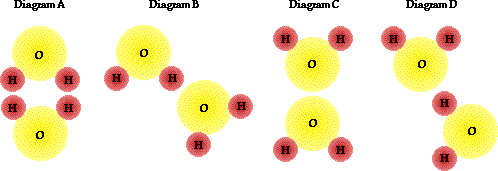
3. The distribution of electric charge in a H2O molecule is nonuniform. The more electronegative oxygen atom attracts electrons from the hydrogen atom. Thus, the oxygen atoms acquire a partial negative charge and the hydrogen atoms acquire a partial positive charge. The water molecule is "polarized." Which diagram(s) below correctly portray(s) a pair of H2O molecules? Explain.
4. True or False:
When an object becomes polarized, it acquires a charge and becomes a charged object.
5. Charged rubber rods are placed near a neutral conducting sphere, causing a redistribution of charge on the spheres. Which of the diagrams below depict the proper distribution of charge on the spheres? List all that apply.

6. In the above situation, the conducting sphere is ____. List all that apply.
|
a. charged
|
b. uncharged (neutral)
|
c. polarized
|